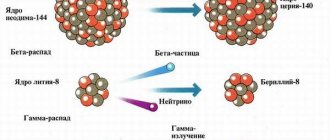
October 2, 2018
The normal functioning of all vital systems depends on the amount of carbon dioxide in the human bloodstream. Carbon dioxide increases the body's resistance to bacterial and viral infections and participates in the metabolism of biologically active substances.
During physical and intellectual stress, carbon dioxide helps maintain the body's balance. But a significant increase in this chemical compound in the surrounding atmosphere worsens human well-being. The harm and benefits of carbon dioxide for the existence of life on Earth have not yet been fully studied.
Characteristics of carbon dioxide
Carbon dioxide, carbonic anhydride, carbon dioxide is a gaseous chemical compound that is colorless and odorless. The substance is 1.5 times heavier than air, and its concentration in the Earth's atmosphere is approximately 0.04%. A distinctive feature of carbon dioxide is that it does not form a liquid when pressure is increased - the compound immediately turns into a solid state known as “dry ice”. But when certain artificial conditions are created, carbon dioxide takes the form of a liquid, which is widely used for its transportation and long-term storage.
Carbon dioxide does not become a barrier to ultraviolet rays that enter the atmosphere from the Sun. But the infrared radiation of the Earth is absorbed by carbon anhydride. This is what causes global warming since the formation of a huge number of industrial productions.
During the day, the human body absorbs and metabolizes about 1 kg of carbon dioxide. It takes an active part in the metabolism that occurs in soft, bone, and joint tissues, and then enters the venous bed. With the blood flow, carbon dioxide enters the lungs and leaves the body with each exhalation.
The chemical is found in the human body primarily in the venous system. The capillary network of pulmonary structures and arterial blood contain a small concentration of carbon dioxide. In medicine, the term “partial pressure” is used, which characterizes the concentration ratio of a compound in relation to the entire volume of blood.
Receiving[ | ]
- In industrial quantities, carbon dioxide is released from flue gases, or as a by-product of chemical processes, for example, during the decomposition of natural carbonates [13] (limestone, dolomite) or during the production of alcohol (alcoholic fermentation). The mixture of the resulting gases is washed with a solution of potassium carbonate, which absorbs carbon dioxide, turning into bicarbonate. A solution of bicarbonate decomposes when heated or under reduced pressure, releasing carbon dioxide. In modern installations for the production of carbon dioxide, instead of bicarbonate, an aqueous solution of monoethanolamine is more often used, which, under certain conditions, is capable of absorbing CO 2 {\displaystyle {\ce {CO2}}} contained in the flue gas, and releasing it when heated; This separates the finished product from other substances.
- Carbon dioxide is also produced in air separation plants as a by-product of the production of pure oxygen, nitrogen and argon.
Kipp apparatus
In laboratory conditions, small quantities are obtained by reacting carbonates and bicarbonates with acids, for example marble, chalk or soda with hydrochloric acid, using, for example, a Kipp apparatus [13]:
CaCO 3 + 2 HCl ⟶ CaCl 2 + H 2 O + CO 2 ↑ {\displaystyle {\ce {CaCO3 + 2HCl -> CaCl2 + H2O + CO2 ^}}} .
Using the reaction of sulfuric acid with chalk or marble results in the formation of slightly soluble calcium sulfate, which slows down the reaction, and which is removed by a significant excess of acid to form acidic calcium sulfate.
To prepare dry drinks, the reaction of baking soda with citric acid or sour lemon juice can be used. It was in this form that the first carbonated drinks appeared. Pharmacists were engaged in their production and sale.
Also, to produce carbon dioxide, an exothermic reaction of combustion of carbon in oxygen is used [13]:
C + O 2 ⟶ CO 2 ↑ + 394 kJ {\displaystyle {\ce {C + O2 -> CO2 ^ + 394 kJ}}} .
Therapeutic properties of carbon dioxide
The penetration of carbon dioxide into the body causes a respiratory reflex in a person. An increase in the pressure of a chemical compound provokes thin nerve endings to send impulses to the receptors of the brain and/or spinal cord. This is how the processes of inhalation and exhalation occur. If the level of carbon dioxide in the blood begins to rise, the lungs accelerate its release from the body.
Scientists have proven that the significant life expectancy of people living in high mountains is directly related to the high content of carbon dioxide in the air. It improves immunity, normalizes metabolic processes, and strengthens the cardiovascular system.
In the human body, carbon dioxide is one of the most important regulators, acting as a main product along with molecular oxygen. The role of carbon dioxide in human life is difficult to overestimate. The main functional features of the substance include the following:
- has the ability to cause persistent dilation of large vessels and capillaries;
- can have a sedative effect on the central nervous system, provoking an anesthetic effect;
- takes part in the production of essential amino acids;
- stimulates the respiratory center with increasing concentration in the bloodstream.
If there is an acute deficiency of carbon dioxide in the body, then all systems are mobilized and increase their functional activity. All processes in the body are aimed at replenishing carbon dioxide reserves in tissues and the bloodstream:
- the vessels narrow, bronchospasm of the smooth muscles of the upper and lower respiratory tract, as well as blood vessels, develops;
- bronchi, bronchioles, structural parts of the lungs secrete an increased amount of mucus;
- the permeability of large and small blood vessels and capillaries decreases;
- Cholesterol begins to deposit on cell membranes, which causes their compaction and tissue sclerosis.
beauty and health
However, CO2 also has a positive effect on the human body. So carbon dioxide is a very powerful disinfectant. It is used in medicine and cosmetology. Carbon dioxide is used together with other components, externally, and also injections are made (Carboxy therapy). A cream or gel containing carbon dioxide disinfects and cleanses the skin well, and its direct introduction into the internal tissues of the body helps fight cellulite.
Inhaling air with a high carbon dioxide content within certain limits or holding your breath also leads to rejuvenation and delays the aging process at the cellular level. The increased CO2 content in arterial blood contributes to the dilation of blood vessels and, as a result, a better and more complete supply of oxygen to the body’s cells.
How does carbon dioxide affect the human body?
As a food additive, carbon dioxide is recognized as “conditionally safe” and is approved for use in almost all countries of the world, including Russia. However, according to experts, excessive consumption, for example in carbonated drinks, of carbon dioxide, the harm of which lies in its ability to increase intestinal absorption, can lead to the following unpleasant consequences:
- rapid intoxication as a result of drinking carbonated alcoholic drinks;
- bloating and belching;
- There is evidence that highly carbonated drinks can leach calcium from bones.
Doctors strongly recommend that people suffering from gastrointestinal diseases stop drinking carbonated drinks.
Although carbon dioxide is non-toxic, elevated concentrations in the inhaled air can be dangerous. With a slight increase in carbon dioxide levels, a person feels weak and drowsy, but if symptoms such as suffocation, dizziness, hearing impairment, or even loss of consciousness are observed, the concentration of carbon dioxide in the air is excessive. The harm of carbon dioxide in this case will consist of hypercapnia (a condition when the concentration of carbon dioxide in the blood increases sharply), which can even lead to death from suffocation.
Harm of carbonic anhydride
Children and adults love a variety of fizzy drinks because of the air bubbles they contain. These accumulations of air are pure carbon dioxide, released when the bottle cap is unscrewed. Used in this capacity, it does not bring any benefit to the human body. Once in the gastrointestinal tract, carbonic anhydride irritates the mucous membranes and provokes damage to epithelial cells.
For a person with stomach diseases, drinking carbonated drinks is extremely undesirable, since their influence intensifies the inflammatory process and ulceration of the inner wall of the digestive system.
Gastroenterologists prohibit patients with the following pathologies from drinking lemonade and mineral water:
- acute, chronic, catarrhal gastritis;
- stomach and duodenal ulcers;
- duodenitis;
- decreased intestinal motility;
- benign and malignant neoplasms of the gastrointestinal tract.
It should be noted that according to WHO statistics, more than half of the inhabitants of planet Earth suffer from one form or another of gastritis. The main symptoms of stomach disease: sour belching, heartburn, bloating and pain in the epigastric region.
If a person is unable to refuse to drink drinks with carbon dioxide, then he should opt for slightly carbonated mineral water.
Experts advise eliminating lemonades from your daily diet. After statistical studies, the following diseases were identified in people who drank sweet water with carbon dioxide for a long time:
- caries;
- endocrine disorders;
- increased fragility of bone tissue;
- fatty liver;
- formation of stones in the bladder and kidneys;
- disorders of carbohydrate metabolism.
Employees of office premises that are not equipped with air conditioning often experience excruciating headaches, nausea, and weakness. This condition occurs in humans when there is an excessive accumulation of carbon dioxide in the room. Constantly being in such an environment leads to acidosis (increased blood acidity) and provokes a decrease in the functional activity of all vital systems.
Notes[ | ]
- Carbon Dioxide - Thermophysical Properties
- Carbon dioxide: Immediately Dangerous to Life or Health Concentrations (IDLH)
- ↑ 12
https://www.cdc.gov/niosh/npg/npgd0103.html - Rakov E. G.
, Carbon dioxide, 2020. - Trends in Atmospheric Carbon Dioxide (English). National Oceanic and Atmospheric Administration. Retrieved September 24, 2013.
- Chen Zhou, Mark D. Zelinka & Stephen A. Klein.
Impact of decadal cloud variations on the Earth's energy budget. Nature Geoscience. Retrieved December 4, 2020. - Egorov A. S.
Chemistry tutor - Rostov-on-Don: “Phoenix”, 2009. - 7. How much carbon dioxide do humans contribute through breathing?. Frequent Questions - Emissions (English). US EPA
. Retrieved December 4, 2020. Archived February 2, 2011. - Charles Henrickson.
Chemistry (undefined). — Cliffs Notes, 2005. — ISBN 0-7645-7419-1. - ↑ 1234
Converted from values in mm. rt. Art. using a conversion factor of 0.133322 kPa/mm. rt. Art. - ↑ 12
Table of reference values. University of Dallas Southwestern Medical Center. - ↑ 1 2 3 4
Carbon dioxide
(undefined)
(inaccessible link).
solarnavigator.net
. Retrieved October 12, 2007. Archived September 14, 2008. - ↑ 1 2 3 Glinka, Nikolaj Leonidovič (1882-1965).
Obŝaâ himiâ. —Izd. 27th ster. - Leningrad: “Himiâ”, 1988. - 702, [2] s. With. — ISBN 5724500035, 9785724500036. - Great Encyclopedia of Oil and Gas.
- GOST 31371.6-2008 (ISO 6974-6:2002). Natural gas. Determination of composition by gas chromatography with uncertainty assessment. Part 6. Determination of hydrogen, helium, oxygen, nitrogen, carbon dioxide and hydrocarbons C1 - C8 using three capillary columns (Russian). Docs.cntd.ru.
_
- M.: Standartinform, 2009. . Retrieved December 4, 2020. - Byalko A.V.
Plants accelerate growth // Nature.
- 1996. - No. 10. (according to Keeling CD, Whorf T.P., Wahlen M., van der Plicht J. // Nature. 1995. V. 375, No. 6533. P.666-670
) - https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5380556/
- Greenwood, Veronica.
Is Conference Room Air Making You Dumber? : [English] // The New York Times: gas. — 2020. — May 6. - Ventilation rates and carbon dioxide concentrations in schools. — In: Ventilation with Outdoor Air: [English] // Berkeley Lab: [website]. — 2020.
- Sorokin, Andrey.
“Global warming is mind-numbing. Schoolchildren and office workers are already suffering from this” // Republic: [website]. — 2020. — January 7. - Indoor Air Quality in Commercial and Institutional Buildings
- (English) Carbon Dioxide as a Fire Suppressant: Examining the Risks, US Environmental Protection Agency:.
- The manufacturer of dry ice spoke out about its toxicity after the deaths of the Russians.
- Acceptable and hazardous levels of carbon dioxide (CO2) concentrations according to ASHRAE and OSHA - USA. Recommended levels of room ventilation.
- (English) Glatte Jr HA, Motsay GJ, Welch BE
Carbon Dioxide Tolerance Studies (unspecified) // Brooks AFB, TX School of Aerospace Medicine Technical Report. - 1967. - T. SAM-TR-67-77. Archived from the original on May 9, 2008.
Sugar or sweeteners
What else is added to carbonated drinks?
Of course, sugar. In itself, it not only causes harm to our body. These are pure carbohydrates that saturate our cells with energy. But you need to remember that sugar in large quantities is harmful. It is bad for the skin, teeth and contributes to weight gain. However, nowadays you rarely see a drink containing sugar. The fact is that it is much more profitable for manufacturers to use sugar substitutes. They come in different types, and we can talk about them for a very long time. But if the packaging contains substances such as cyclamate (E 952), saccharin (E 954), aspartame (E 951) or sucrasite, you should not drink such lemonade. Firstly, some of these substances are banned in Europe and America. Studies have shown that they have a negative effect on the liver and kidneys, and also contribute to the development of various diseases, including cancer. Secondly, sweeteners make you hungry. Therefore, soda contributes to excess weight gain. Even the so-called “diet cola” is an enemy to our figure, because it improves appetite.
There are drinks that use plant components as sweeteners - sorbitol, xylitol and fructose. They are absolutely harmless, but very high in calories. Therefore, if you are not afraid of gaining excess weight, you can drink lemonades with sugar or natural sweeteners.
The dangers of “fizzy” water for a child
In recent years, nutritionists and pediatricians have been sounding the alarm. Parents are increasingly buying carbonated drinks for their young children. The consequences of such unreasonable actions are obvious: the number of boys and girls suffering from obesity is steadily growing every year. What can abuse of soda lead to? Increased nervous excitability, problems with the skeletal and endocrine systems, bad teeth. All this is just a small part of the harm that sweet carbonated water can have on the body.
In addition to children, pregnant women and nursing mothers, as well as people who are struggling with excess weight, diseases of the gastrointestinal tract, and allergy sufferers should avoid sweet soda.
Production of carbonated drinks
The basis of any lemonade is water. Therefore, during the production of the drink, special requirements are placed on its quality. Global manufacturers ensure that water at their factories undergoes thorough, multi-stage purification. After all, the quality of this liquid affects the taste of the drink, its aroma and, of course, the health of the buyer. First, all small particles are removed from the water. After all impurities are eliminated, it becomes perfectly transparent. This is the first stage of filtration.
Then the water goes through several more stages of purification until its properties meet all requirements and standards. The very last stage is the passage of water through a carbon filter. This procedure allows you to remove the smallest particles, and even germs and bacteria. Thanks to it, water acquires excellent taste and aromatic properties. To remove particles of coal that accidentally fall into the water, it is additionally passed through a polishing filter. After this, the water can be used to prepare any drink.
The next important ingredient in lemonade is syrup. It is this that gives the unique taste and aroma to the drink. Each company has its own unique syrup recipe. Global manufacturers, with branches in hundreds of countries, send the concentrate in closed containers so that no one can find out the secret formula.
The finished concentrate is mixed with white sugar syrup in the blending department. And the finished mixture is sent to the workshop where the lemonade is actually made. But before this, the syrup must undergo quality testing in a specialized laboratory. It must meet not only the manufacturer’s internal requirements, but also international standards.
In the bottling workshop, carbon dioxide is injected into the water, mixed with syrup and bottled. After this, all products undergo a control system. Bottles with crookedly glued stickers, underfilled or overfilled lemonade are sent to waste.
Literature[ | ]
- Vukalovich M. P., Altunin V. V.
Thermophysical properties of carbon dioxide. - M.: Atomizdat, 1965. - 456 p. - Grodnik M. G., Velichansky A. Ya.
Design and operation of carbon dioxide plants. - M.: Food Industry, 1966. - 275 p. - Rakov E. G.
Carbon dioxide (Russian) // Great Russian Encyclopedia. - M.: Great Russian Encyclopedia, 2020. - T. 32. - P. 662-663. - Tezikov A.D.
Production and use of dry ice. - M.: Gostorgizdat, 1960. - 128 p. - Talyanker Yu. E.
Features of storage of liquefied gas cylinders // Welding production. - 1972. - No. 11.
First aid
First aid for carbon dioxide poisoning is to stop contact with carbon dioxide. To do this, the victim should be taken out into fresh air.
First aid technique, according to WHO recommendations:
- Provide a constant supply of fresh air.
- If the victim is unconscious, then lay him on his side. This will prevent vomit from entering the respiratory system.
- Do not leave the victim unattended until the ambulance arrives. Drink warm tea.
- If breathing stops, perform resuscitation measures.
- Call an emergency team.
The doctor will decide on hospitalization depending on the condition of the victim. Mild intoxication does not require hospital stay. You can get by with treatment at home.
Victims with moderate and severe forms of poisoning. If a child is injured, regardless of pathogenesis, they must be taken to the clinic.
What information is missing from the article?
- List of effective medications
- A detailed overview of traditional methods of treatment
- Professional opinion of a specialist
- Detailed review of antidotes