Invisible rays penetrate through all objects around and through ourselves. We do not perceive or feel them in any way. It is impossible to defend against them; they are elusive and pervasive. They can heal and they can kill, they can contribute to the birth of previously unseen creatures on earth and lead to the emergence of new star clusters in the distant corners of our galaxy.
Ionizing radiation
All this is not a fragment of the ravings of a madman, taken from the history of his illness, and not a brief synopsis of another Hollywood action movie. This is the reality surrounding us, which is called radioactive or ionizing radiation, in short - radiation.
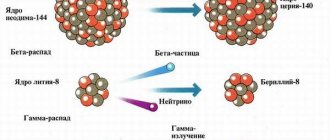
The phenomenon of radioactivity was formulated in general terms by the French physicist A. Becquerel in 1896. E. Rutherford concretized this phenomenon and described it in more detail in 1899. It was he who was able to establish that radioactive radiation is heterogeneous in nature and consists of at least three types of rays. These rays were deflected differently in the magnetic field and therefore received different names. The penetrating power of alpha, beta and gamma radiation is different.
Alpha rays
In a magnetic field, they are deflected in the same way as positively charged particles. Later it was found out that these are heavy, positively charged nuclei of helium atoms. They occur during the decay of more complex atomic nuclei, such as uranium, radium or thorium. They have a large mass and a relatively low radiation speed. This determines their low penetrating ability. They cannot penetrate even a sheet of paper.
But at the same time, alpha particles have very high ionizing energy, which is the reason for their ability to cause very serious damage at the cellular level. Of all types of rays, it is alpha rays that are characterized by the most severe consequences if they affect the body.
This destructive effect occurs only in the case of direct contact with objects emitting alpha rays. In practice, this occurs as a result of radioactive elements entering the body through the gastrointestinal tract when eating food or water, as well as when inhaling air saturated with radioactive dust. In addition, alpha particles can easily enter the body through damaged skin. Carrying through the bloodstream throughout the body, they have the ability to accumulate, exerting a strong destructive effect for many years.
It must be borne in mind that radioactive substances entering the body are not removed from it on their own. The human body is practically not protected from this kind of penetration. It cannot neutralize, process, assimilate or remove on its own a radioactive isotope that gets inside.
Beta rays
They are deflected in the same direction as negatively charged particles. The source of beta radiation is intranuclear processes associated with the transformation of a proton into a neutron and vice versa - a neutron into a proton. In this case, an electron or positron is emitted. The propagation speed is quite high and approaches the speed of light. Beta radiation has a much greater penetrating power than alpha radiation, but the ionizing effect is much less pronounced.
Beta radiation easily penetrates clothing, but a thin sheet of metal or a medium-thick block of wood stops it completely. Unlike alpha radiation, beta rays are capable of causing remote damage at a distance of several tens of meters from the radiation source.
Gamma rays
These rays turned out to be neutrally charged and were not deflected in any way in the magnetic field. Gamma radiation is electromagnetic energy emitted in the form of photons. This energy is released when the energy state of the atomic nucleus changes.
This type of radiation is characterized by high speed, equal to the speed of light, and extremely high penetrating ability. Thick concrete walls are needed to stop gamma radiation. The paradox is that this type of rays is least likely to have a destructive effect on the body. Their ionizing effect is hundreds of times weaker than beta radiation and tens of thousands of times weaker than alpha radiation. But the ability to travel significant distances and high penetrating properties make these rays potentially the most dangerous to humans. Therefore, let us dwell on this type of radiation in more detail.
Biological effects[ | ]
Irradiation with gamma quanta, depending on the dose and duration, can cause chronic and acute radiation sickness. Stochastic effects of radiation include various types of cancer. At the same time, gamma irradiation suppresses the growth of cancer and other rapidly dividing cells when applied locally. Gamma radiation is a mutagenic and teratogenic factor.
Defense[ | ]
A layer of substance can serve as protection against gamma radiation. The effectiveness of protection (that is, the probability of absorption of a gamma quantum when passing through it) increases with increasing thickness of the layer, density of the substance and the content of heavy nuclei in it (lead, tungsten, depleted uranium, etc.).
The table below shows the parameters of the half-attenuation layer[en] of gamma radiation with an energy of 1 MeV for various materials:
| Protection material | Density, g/cm³ | Half attenuation layer, cm | Weight of 1 cm² half-attenuation layer, g |
| Lead | 11,35[7] | 0,8[8][7][9][10] | |
| Concrete | 1,5-3,5[11] | 3,8-6,9[11] | |
| Steel | 7,5-8,05[12] | 1,27[8] | |
| Iron | 7,86[7] | 1,5[7][9] | |
| Aluminum | 2,82[7] | 4,3[7][10] | |
| Tungsten | 19,3[13] | 0,33[8] | |
| Water | 1,00[7] | ~10[7][9][10] | |
| Depleted uranium | 19,5[14] | 0,28[8] | |
| Air | 0,0013[7] | ~8500[7][10] |
Although absorption efficiency depends on the material, the primary consideration is simply the specific gravity.
Gamma radiation
It is a type of electromagnetic radiation. It has a very short wavelength. As a result of this, its corpuscular properties are strongly expressed and its wave properties are extremely weakly expressed. The short wavelength determines the very large amount of energy inherent in this type of radiation. Belongs to the so-called group of ionizing radiation, which also includes alpha, beta, x-rays and neutrons. At the same time, visible light and ultraviolet radiation are not ionizing, like infrared or radio radiation. Gamma radiation is a stream of neutral particles in the form of electromagnetic waves.
Ionization process
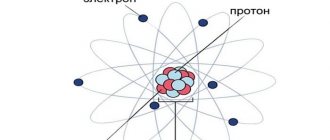
In the normal state, the intraatomic nucleus and the electrons rotating around it are a fairly stable system with a neutral charge, since the positive charge of the atom is balanced by the negative charge of the electrons. To disrupt this balance, one or more electrons must be knocked out of the atom. As a result, the atom ceases to be neutral and acquires a certain charge or quantum, which can be either positive or negative.
The atom becomes an ion with a corresponding charge, and the process of knocking electrons out of the atom is called the ionization process.
Radiation or ionizing radiation is a stream of particles that are capable of knocking out electrons from an atom and thereby giving it special properties that differ sharply from ordinary ones.
Destructive effects of radiation
Radiation is primarily understood as gamma radiation, the properties of which make it the most dangerous of all existing types. The destructive effect is manifested in the following:
It leads to the formation of ions, which, in turn, themselves become sources of ionization. A kind of chain reaction occurs that is extremely difficult to stop.
- Under the influence of radiation, destruction occurs at the molecular level, which leads to the formation of endogenous poisons that begin to poison the body from the inside.
- Gene mutations increase many times over, leading to the emergence of pathologically altered cellular neoplasms.
- Cells capable of rapid division are most susceptible to damage. As a result, gene mutations are quickly transmitted to new generations of cells.
- The hematopoietic, digestive and reproductive systems are primarily affected.
Radiation sources
Several potentially dangerous sources of gamma radiation can be identified. Some of them existed long before the advent of man and still exist, and some are artificially created by man himself for his needs:
- External natural sources. Cosmic rays and solar radiation. Sources on the surface of the earth in certain places where radioactive rocks occur.
- Internal sources - enter our body with water or food, as well as as a result of inhalation of radioactive dust.
- External artificial sources. All are products of modern technogenic civilization. These are nuclear energy enterprises, mining plants specializing in the extraction and enrichment of uranium. This also includes devices and instruments that contain a certain amount of radioactive substances and are potential emitters.
High levels of radiation can be observed in high altitude areas, near active volcanoes, in airliner cabins when flying at high altitudes, or in spacecraft.
It must be borne in mind that there is a certain safety margin within which the body is able to feel comfortable without experiencing the negative effects of radiation. This reserve is individual for each individual person.
Peaceful atom
Like any complex and ambiguous natural phenomenon, radiation carries with it not only death, death and destruction, but also benefits. Gamma rays have found very wide application in everyday human life:
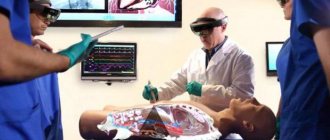
- In medicine, highly effective sterilization of instruments and dressings is performed.
- The property of gamma rays to cause deep ionization and subsequent destruction of living cells has found wide application in oncology. Cancer cells are characterized by uncontrolled, chaotic division and corresponding growth. In this case, gamma radiation has found application as a weapon of last chance, when other methods are powerless and have a destructive effect on these types of cells.
- In high-precision industries, for example, in space, gamma rays are used to check hidden defects in metal products.
- In the mining industry, the depth of rocks is measured and the drilling depth is subsequently determined.
- In agriculture, with the help of a strictly dosed flow of gamma rays aimed at the seeds of certain plants, artificial mutations are caused in order to produce plants with new properties - for example, resistant to drought or low temperatures.
- Gamma rays are used to determine the trajectory, speed and distances during piloting of spacecraft.
Protection from harmful effects
As a rule, all natural sources of increased radiation do not pose a particular danger to humans due to their inaccessibility. A much greater danger is posed by artificially created sources, such as nuclear power plants, uranium mining and enrichment enterprises, and household items containing certain substances that emit gamma rays.
At all enterprises associated with increased background radiation, the following protective measures are implemented:
The time and frequency of contact with the radiation source is strictly regulated.
- To work in a radiation source, special protective clothing is used, and upon completion of work they undergo a multi-level intoxication system.
- When constructing buildings in or near a source of radiation, special materials are used that effectively block this type of radiation. Such materials include high-strength reinforced concrete, pure lead and lead glass, and some types of special steels.
- When working in areas with both high radiation and high temperature, for example, in a fusion reactor, lead cannot be used because it has a low melting point, so in these cases more expensive rare earth metals are used, such as tungsten and tantalum.
Using[ | ]
| This section is missing references to information sources. Information must be verifiable, otherwise it may be questioned and deleted. You may edit this article to include links to authoritative sources. This mark was set on October 29, 2020 . |
Areas of application of gamma radiation:
- Gamma flaw detection - inspection of products by transillumination with γ-rays.
- Food industry: food preservation (gamma sterilization to increase shelf life)[6].
- Medicine: sterilization of medical materials and equipment; radiation therapy; radiosurgery.
- Gamma ray logging in geophysics.
- Instruments for measuring distances: level gauges, gamma altimeters on spacecraft.
- Gamma-ray astronomy.
Methods of protection
The Earth has a natural defense mechanism against cosmic radiation: the ozone layer and the upper atmosphere.
Those rays that, having enormous speeds, penetrate into the protected space of the earth do not cause much harm to living beings. The greatest danger comes from sources and gamma radiation received in terrestrial conditions.
The most important source of danger from radiation contamination remains enterprises where controlled nuclear reactions are carried out under human control. These are nuclear power plants where energy is produced to provide the population and industry with light and heat.
The most serious measures are being taken to provide for the workers of these facilities. The tragedies that occurred in different parts of the world, due to the loss of human control over the nuclear reaction, taught people to be careful with the invisible enemy.
Notes[ | ]
- D. P. Grechukhin.
Gamma radiation // Physical encyclopedia: [in 5 volumes] / Ch. ed. A. M. Prokhorov. - M.: Soviet Encyclopedia (vol. 1-2); Great Russian Encyclopedia (vol. 3-5), 1988-1999. — ISBN 5-85270-034-7. - RMG 78-2005. Ionizing radiation and its measurements. Terms and concepts. M.: Standartinform, 2006.
- According to practical transcription, the correct way to transfer the surname is Villar
, but this option is not found in the sources. - ↑ 123
The discovery of gamma rays Archived March 16, 2005. (English) - Gerward L.
Paul Villard and his Discovery of Gamma Rays // Physics in Perspective. - 1999. - Vol. 1. - P. 367-383. - A program of gamma sterilization of agricultural products is planned in the Russian Federation (Russian). RIA Novosti (September 28, 2010). Retrieved September 28, 2010. Archived August 25, 2011.
- ↑ 12345678910
Half Value Layers (in cm) for Gamma and X-Ray Radiations at Varying Energies for Various Materials. Snow College. Retrieved February 4, 2020. - ↑ 1 2 3 4
Half-Value Layer
(undefined)
. NDT Resource Center. Retrieved February 4, 2020. - ↑ 123
Shielding Radiation - Alphas, Betas, Gammas and Neutrons. USA NRC (7/05/2011). Retrieved February 5, 2020. - ↑ 1 2 3 4
Absorption of γ-rays – Determination of the Half-value Thickness of Absorber Materials
(undefined)
.
Laboratory Manuals for Students in Biology and Chemistry - Course PHY117
. Physik-Institut der Universität Zürich. Retrieved February 10, 2020. - ↑ 1 2 A. Akkaş.
Determination of the Tenth and Half Value Layer Thickness of Concretes with Different Densities: [English] // Acta Physica Polonica A. - 2020. - T. 129, no. Special Issue of the 5th International Advances in Applied Physics and Materials Science Congress & Exhibition APMAS2015, Lykia, Oludeniz, Turkey, April 16-19, 2020, No. 4 (April). — P. 770-772. - doi:10.12693/APhysPolA.129.770. - Elert, Glenn
Density of Steel
(unspecified)
. Retrieved April 23, 2009. - Tungsten (English). Midwest Tungsten Service. Retrieved February 11, 2020.
- Brian Littleton.
Depleted Uranium Technical Brief. US Environmental Protection Agency (Dec. 2006). Retrieved February 11, 2020.
Where else does γ decay occur?
But ionizing radiation does not only occur during radioactive decay. They also occur during atomic explosions and in nuclear reactors. On the Sun and other stars, as well as in a hydrogen bomb, the synthesis of light nuclei occurs, accompanied by ionizing radiation. This process also occurs in X-ray equipment. The main property that alpha, beta, and gamma decays have is the highest ionization energy.
And the differences between these three types of radiation are determined by their nature. Radiation was discovered at the end of the 19th century. At that time no one knew what this phenomenon was. Therefore, the three types of radiation were named by letters of the Latin alphabet. Gamma radiation was discovered in 1910 by a scientist named Henry Gregg. Gamma decay is of the same nature as sunlight, infrared rays, and radio waves. In terms of their properties, γ-rays are photon radiation, but the energy of the photons they contain is very high. In other words, it is radiation with a very short wavelength.