Education[ | ]
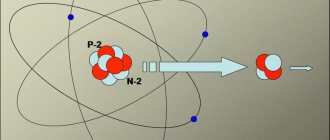
Alpha particles arise from the alpha decay of nuclei, during nuclear reactions and as a result of the complete ionization of helium-4 atoms. For example, as a result of the interaction of a lithium-6 nucleus with a deuteron, two alpha particles can be formed: 6Li
+
2H
=
4He
+
4He
. Alpha particles make up a significant portion of primary cosmic rays; most of them are accelerated helium nuclei from stellar atmospheres and interstellar gas, some arose as a result of nuclear spallation reactions from heavier cosmic ray nuclei. High energy alpha particles can be produced using charged particle accelerators.
Alpha radiation
Some radionuclides with high atomic mass (Ra226, U238, Pu239) decay under the influence of alpha particle radiation. These alpha particles are tightly bound units of two neutrons and two protons each (He4 nucleus) and have a positive charge. The emission of an alpha particle from the nucleus results in a decrease of two units of atomic number (Z) and four units of mass number (A). Alpha particles are emitted with discrete energies characteristic of the particular transformation from which they originate. All alpha particles from a particular radionuclide transformation will have the same energy.
Penetration ability[ | ]
Heavy charged particles interact mainly with atomic electrons and therefore deviate little from the direction of their initial motion. As a result, the range of a heavy particle R
measured by the distance in a straight line from the source of the particles to the point where they stop.
Typically, the run is measured in units of length (m, cm, microns), as well as the surface density of the material (or, equivalently, the run length multiplied by the density) (g/cm2). Expressing the distance in units of length makes sense for a fixed density of the medium (for example, dry air is often chosen as the medium under normal conditions). The physical meaning of the path in terms of surface density is the mass per unit area of the layer sufficient to stop the particle. The path length of an α-particle depending on its energy and environment
| Wednesday | Energy of α-particles, MeV | |||
| 4 | 6 | 8 | 10 | |
| Path length of α-particle, mm | ||||
| Air under normal conditions | 25 | 46 | 74 | 106 |
| Biological tissue | 0,031 | 0,056 | 0,096 | 0,130 |
| Aluminum | 0,016 | 0,030 | 0,048 | 0,069 |
Isotope decay rate (half-life)
Each radionuclide decays at its own unique rate, which cannot be changed by any chemical or physical process. A useful indicator of this rate is the half-life of the radionuclide.
Half-life is defined as the time required for the activity of any particular radionuclide to decrease to half its original value. In other words, half of the atoms returned to a more stable state of the material.
Half-lives of radionuclides range from microseconds to billions of years.
The half-lives of two widely used industrial isotopes are 74 days for iridium-192 and 5.3 years for cobalt-60. More accurate calculations can be made for the half-lives of these materials, however, these times are generally used.
Note that carbon-14 is not used in radiography, but is one of many useful radioactive isotopes used to determine the age of fossils.
Detection[ | ]
Alpha particles are detected using scintillation detectors, gas discharge detectors, silicon pin diodes (surface barrier detectors insensitive to beta and gamma radiation) and associated amplification electronics, as well as track detectors. To detect alpha particles with energies characteristic of radioactive decay, it is necessary to ensure a low surface density of the screen separating the sensitive volume of the detector from the environment. For example, gas-discharge detectors can use a mica window several microns thick that is permeable to alpha particles. In semiconductor surface-barrier detectors such a screen is not needed; the working area of the detector can be in direct contact with air. When detecting alpha-active radionuclides in liquids, the test substance is mixed with a liquid scintillator.
Currently, the most common are silicon surface barrier detectors of alpha particles, in which on the surface of a semiconductor crystal with conductivity p
-type, a thin layer with
n
-type conductivity is created by diffusion introduction of a donor impurity (for example, phosphorus).
Applying a reverse bias to the pn
junction depletes the sensitive area of the detector with charge carriers. The entry of an alpha particle that ionizes a substance into this region causes the birth of several million electron-hole pairs, which cause a recorded current pulse with an amplitude proportional to the number of pairs produced and, accordingly, to the kinetic energy of the absorbed alpha particle. Since the depletion region is very thin, the detector is sensitive only to particles with a high ionization density (alpha particles, protons, fission fragments, heavy ions) and is insensitive to beta and gamma radiation.
Types of radiation
Types of radiation caused by radioactive decay. When an atom undergoes radioactive decay, it emits one or more forms of radiation with sufficient energy to ionize the atoms with which it interacts. Read more about the history of the treatment.
Ionizing radiation can consist of high-speed subatomic particles ejected from the nucleus, or electromagnetic radiation (gamma rays) emitted either by the nucleus or orbital electrons.
Impact on humans[ | ]
Alpha particles formed during nuclear decay have an initial kinetic energy in the range of 1.8-15 MeV[3]. When an alpha particle moves in a substance, it creates strong ionization of the surrounding atoms, and as a result of this, it very quickly loses energy. The energy of alpha particles resulting from radioactive decay is not enough to even penetrate the dead layer of skin, so there is no radiation risk from external exposure to such alpha particles. External alpha radiation is dangerous to health only in the case of high-energy alpha particles (with energies above tens of MeV), the source of which is an accelerator. However, the penetration of alpha-active radionuclides into the body, when living tissues of the body are directly exposed to irradiation, is very dangerous for health, since the high ionization density along the particle track severely damages biomolecules. It is believed [4] that with equal energy release (absorbed dose), the equivalent dose accumulated during internal irradiation with alpha particles with energies characteristic of radioactive decay is 20 times higher than during irradiation with gamma and x-ray quanta. However, it should be noted that the linear energy transfer of high-energy alpha particles (with energies of 200 MeV and above) is much less, so their relative biological effectiveness is comparable to that of gamma rays and beta particles.
Thus, the danger to humans from external
irradiation can represent α-particles with energies of 10 MeV and higher, sufficient to overcome the dead stratum corneum of the skin. At the same time, most research α-particle accelerators operate at energies below 3 MeV[5].
A much greater danger to humans is represented by α-particles arising from the alpha decay of radionuclides that enter
body (in particular, through the respiratory tract or digestive tract)[6]. A microscopic amount of an α-radioactive substance (for example, polonium-210) is enough to cause acute radiation sickness in a victim, often with a fatal outcome[6].
What is radioactivity in physics
Any atom has a nucleus and negative charged particles - electrons - rotating around it.
The atomic nucleus consists of protons and neutrons. Moreover, the number of protons is always the same and corresponds to the serial number of the chemical element in the periodic system of Mendeleev. Nuclei in which the number of neutrons differs are called isotopes.
Some atomic nuclei can transform into different isotopes, releasing elementary particles or light nuclei. Actually this process is called radioactivity.
This phenomenon can be defined as follows: the ability of an atomic nucleus to decay uncontrollably with the emission of penetrating particles.
Nuclear decay is possible if it is accompanied by the release of energy. Today, about 3 thousand atomic nuclei are known. Of these, only 264 are non-radioactive.
In physics, there are the following types of radioactive decay:
- α-decay with the release of an α-particle;
- β-decay with the emission of an electron and an antineutrino, a positron and a neutrino, as well as the absorption of an electron by the nucleus with the release of a neutrino;
- γ-decay – emission of ionizing rays from an atomic nucleus;
- uncontrolled division of the nucleus into fragments.
Answers
Composition of the atomic nucleus. Nuclear reactions →
← Dispersion of light. Lens. Focal length of the lens. The eye as an optical system. Optical instruments
Radioactivity. Alpha, beta, gamma radiation. Rutherford's experiments. Planetary model of the atom
Grade
main sources
Sources of alpha radiation.
The main sources of alpha radiation are:
- Formation of helium isotopes. Observed during the decay of heavy atoms.
- Interstellar gas. It is formed when the speed of movement of helium nuclei in space increases. These particles try to overcome the force of gravity.
- Scientific experiments. Experiments are carried out using accelerators in laboratory conditions. The equipment produces radiation with the required characteristics.
- Industry. The sources are nuclear power and uranium industry facilities.
How does beta radiation affect humans?
Burns occur when the rays come into direct contact with the skin. The severity of damage depends on the duration, intensity and structure of radiation. Most often, the electrons generated during the decay of particles damage the organs of vision and mucous membranes.
When ingested by the respiratory and digestive organs, the elements spread throughout the body.
The process is accompanied by ionization of molecules, release of toxins and cell death. Intoxication developing against the background of radiation sickness causes death.
There are standards that help determine the intensity of radiation exposure. An indicator of 0.20 μSv/h is considered safe.
If the background radiation exceeds the norm by 2 times, you can stay in this area for no more than half an hour.
Application area
The main area of application of this type of radiation is medicine. We are talking about radioisotope diagnostics and treatment of certain diseases.
Practical use is carried out:
- For therapeutic purposes. Applications are applied to the affected areas, emitting particles needed for treatment.
- To eliminate malignant neoplasms. Therapy can be interstitial or intracavitary (a radiation source is introduced into the organ affected by the tumor). The electrons released during radiation decay negatively affect the division processes of cancer cells.
- For diagnostic purposes. The method is based on the accumulation of radioactive isotopes in tumor tissues. Such a study helps to identify the smallest malignant neoplasms.
Beta radiation is also used in the chemical industry, for example in the control of automatically occurring processes. Irradiation is used in the repair of transport and construction equipment, and in archaeological excavations. The use of rays helps determine the exact age of the rock.
Which radionuclides pose the greatest danger?
This is quite a provocative question. On the one hand, short-lived ones are more dangerous, because they are more active. But after their decay, the problem of radiation itself loses relevance, while long-lived ones pose a danger for many years.
The specific activity of radionuclides can be compared to weapons. Which weapon will be more dangerous: one that fires fifty shots per minute, or one that fires once every half hour? This question cannot be answered - it all depends on the caliber of the weapon, what it is loaded with, whether the bullet will reach the target, and what the damage will be.
Units of measurement of radioactivity
However, how is this quantity measured? Measuring radioactivity allows the decay rate to be expressed in numbers. The unit of measurement for radionuclide activity is the becquerel. 1 becquerel (Bq) means that 1 decay occurs in 1 second. Once upon a time, a much larger unit of measurement was used for these measurements - the curie (Ci): 1 curie = 37 billion becquerels.
Naturally, it is necessary to compare identical masses of a substance, for example, 1 mg of uranium and 1 mg of thorium. The activity of a given unit mass of radionuclide is called specific activity. The longer the half-life, the lower the specific radioactivity.