The discovery of radioactivity and its unusual properties aroused great interest in the scientific world and raised many questions. A breakthrough in the study of this phenomenon was the classic experiment of the English scientist E. Rutherford, who placed a radioactive emitter in a magnetic field. To the surprise of the experimenters, the radioactive beam was divided into 3 parts. The rays that experienced minimal deflection were called alpha rays.
What is alpha radiation? What has it become for humanity - a friend, a helper or an enemy? What is the harm from it and how to protect yourself from alpha radiation?
Energy of education
To calculate the energy of alpha particle formation, you should use Einstein's famous equation, which relates mass and energy through one of the fundamental constants of our Universe - the speed of light. This equation has the form: E = mc2, where E is energy, m is mass, c is the speed of light in vacuum.
Knowing that when an alpha particle is formed, the mass of its components decreases by 0.015 * 10-27 kg, and also knowing that the speed of light is 3 * 108 m/s, we obtain the energy that is released during this process. It is equal to E = 0.015 * 10-27 * 9 * 1016 = 1.35 * 10-12 J. In particle physics, it is customary to write energies in electron volts (eV). One electron-volt is equal to 1.602177 * 10−19 J. Then the energy of formation of an alpha particle is equal to 8.426 * 106 eV, or 8.426 MeV (megaelectron-volt).
To understand how big this energy is, you can do a simple calculation. Let's imagine that all the energy of the formation of an alpha particle is transferred to its acceleration. Using the Lorentz equation for non-relativistic velocities, that is, assuming that the kinetic energy of an alpha particle is equal to mv2/2, where v is the speed of its movement, we find that this formation energy will be sufficient to accelerate the alpha particle to a speed of 2 * 107 m/ c, which is 6.7% of the speed of light in vacuum. Note that asking the question of how much the alpha particle’s mass will increase at such speeds does not make sense, since the increase in its mass can be neglected, since it will be only 0.015/6.68 * 100 = 0.2%.
Basic physical properties
An alpha particle is 4 times heavier than a proton and 8000 times heavier than an electron, that is, for the world of elementary particles it has a large mass. Recall that the mass of one proton or one neutron in atomic units (amu) is equal to 1, and the charge of a proton is +1 in units of elementary charge, that is, the alpha particle has a charge of +2 and a mass of 4. Then The charge to mass ratio of an alpha particle is +1/2 = +0.5.
Since it has an electrical charge, when it flies through an electric or magnetic field, it interacts with it. To determine the direction of the force that acts on an alpha particle in a magnetic field, it is necessary to use the so-called left-hand rule: four fingers should be placed along the motion vector of the alpha particle, and the palm should be rotated so that the magnetic induction lines enter it. Then the thumb protruded at a right angle will indicate the direction of the acting force on the moving charged particle.
Alpha particles can accelerate to high speeds, reaching values of 15 million km/s, that is, 5% of the speed of light. Due to their large mass and enormous speeds, they acquire significant kinetic energy, which can be up to 10 MeV.
Completeness
The delivery package for OSAI sources includes the components and operational documentation listed in Table 3.
| Name | Designation | Quantity |
| Source with main radionuclide* | 1 | |
| Pencil case for storing the source | 1 | |
| Certificate of metrological certification (verification) | 1 | |
| Passport to the source | 1 |
Note
*) Sources can be supplied in sets or individual sources; the main radionuclides in the sources and the nominal activity of radionuclides are determined by agreement with the Customer.
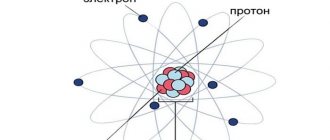
Elementary particles: protons and neutrons
In physics, it is customary to attribute two main characteristics to any particle - electric charge and mass, since these criteria largely determine its properties and behavior under specific physical conditions.
As mentioned above, an alpha particle consists of two protons and two neutrons. A proton is an elementary particle having a mass of 1.6726 * 10-27 kg and a charge of +1.602 * 10-19 C. As for the neutron, its mass is 1.00137 times greater than that of the proton, that is, 1.67489 * 10-27 kg. The charge of a neutron is zero, that is, this particle is electrically neutral (hence the name “neutron”).
Nuclear reactions
Alpha decay
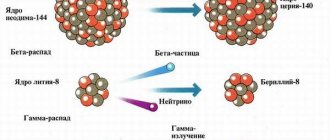
Example: where - alpha radiation - helium nuclei.
This decay is observed for heavy nuclei with A>200. During the alpha decay of one chemical element, another chemical element is formed, which in the periodic table is located 2 cells closer to its beginning than the original one.
Beta decay
Example: where - beta radiation - electrons.
During the beta decay of one chemical element, another chemical element is formed, which is located in the periodic table in the next cell after the original one.
Gamma radiation
The emission of gamma radiation does not lead to transformations of elements.
During a nuclear reaction, the total electric charge and the number of nucleons are conserved. Nuclear reactions are of two types: endothermic (with the absorption of energy) and exothermic (with the release of energy). If the sum of the masses of the initial nucleus and particles is greater than the sum of the masses of the final nucleus and emitted particles, then energy is released, and vice versa.
Discovery of the proton:
Discovery of the neutron:
Ionizing radiation is a stream of particles capable of causing ionization of matter. During ionization, an electron or several electrons are removed from an atom or molecule, which are transformed into positively charged ions. Electrons removed from atoms or molecules can be attached to other atoms or molecules, forming negatively charged ions.
The discharge of a charged electrometer in the air, which occurs regardless of the quality of the electrical insulation of the device, was noticed by Charles Coulomb in 1785, but only in the 20th century was it possible to explain the patterns he discovered by the action of cosmic rays, which are one of the components of natural ionizing radiation.
The result of ionizing radiation is called irradiation. Despite the variety of phenomena that occur in matter under the influence of ionizing radiation, it turned out that irradiation can be characterized by a single quantity called the radiation dose.
The effect of ionizing radiation in a wide range of doses is hidden from the immediate sensations of a person and therefore it seems to him one of the most dangerous exposure factors.
In everyday life and in some branches of science, technology and medicine, ionizing radiation is usually called simply radiation. Strictly speaking, this is not entirely true, because... the term “radiation” itself covers all types of radiation, including the longest radio waves and streams of particles of any arbitrarily low energy, as well as deformation waves in matter, for example, sound waves. However, the use of the word “radiation” in relation to ionizing radiation has become such a habit that terms formed on its basis have taken root in science, such as, for example, radiology (the science of medical applications of ionizing radiation), radiation protection (the science of methods of reducing radiation doses to acceptable levels), natural background radiation, etc.
Alpha particle mass
If we take into account the additive property of the physical quantity “mass,” then we can independently calculate how much an alpha particle weighs. The above figures for protons and neutrons say that the mass of an alpha particle is 6.69498 * 10-27 kg. This figure is obtained if we add up the rest masses of two protons and two neutrons. As a result, the ratio of the masses of the proton and the alpha particle is approximately 1/4. That is, an alpha particle is four times heavier than a proton.
However, many experiments conducted to establish the exact mass of this particle say that the rest mass of the alpha particle is 6.68 * 10 * 10-27 kg, that is, it is 0.015 * 10-27 kg less than the value obtained above. Where does the difference go? The answer to this question is quite simple - it turns into energy. The fact is that when an alpha particle is formed from protons and neutrons as a result of nuclear interactions between them, energy is released in the form of electromagnetic radiation, two protons and two neutrons pass into a more favorable energy state - our alpha particle.
Radiation: sources
In a previous post I talked about the units of measurement of ionizing radiation. Now let's talk about radiation sources. I won’t write here about “what you shouldn’t touch with your hands” - a lot has been written about this, but I’m not Oleg Aizon and I don’t have unique photographs of hitherto unseen radioactive artifacts. I will tell you in general where our radiation comes from.
Radioactive decay as a phenomenon
What is radioactive decay?
Someone, remembering their school knowledge, will answer - this is the phenomenon of transformation of some elements into others. Someone will give a different, usually equally inaccurate, definition. In fact, radioactive decay is any spontaneous change in the state of an atomic nucleus as a system of nucleons, accompanied by the release of energy, the value of which, as a rule, exceeds several kiloelectronvolts. This energy is then carried away by elementary particles emitted from the nucleus, quanta of electromagnetic radiation, or transferred to the electrons of the atom. In this case, the nucleus itself can change its charge, mass, split into two or more nuclei, or it can remain itself, only passing into a more stable state. The “external”, easily determined characteristics of the atomic nucleus are its mass A
and charge (or atomic number)
Z
, measured in proton charges and masses.
These are integer quantities that have the physical meaning of the number of corresponding particles in the nucleus. The charge of a neutron is zero, and the mass is almost the same as that of a proton, so calculate the number of neutrons: . Nuclei with the same charges are called isotopes
, with the same masses -
isobars
, but if both are the same, we are dealing with
isomers
. Z and A are indicated to the left of the element symbol in subscript and superscript, respectively.
From what has been said, it is obvious that in order for Z to change, a charged particle must leave the nucleus, and for A to change, something heavier than an electron must fly away from the nucleus. So, the following options are possible:
- an electron and an antineutrino or a positron and a neutrino (beta decay) are emitted - Z changes by one (increases in the case of electron and decreases in the case of positron decay), A - does not change;
— the nucleus, on the contrary, can absorb an electron from the K-level of the atom (K-capture) — Z increases by one (as in beta-plus decay), A does not change, a neutrino is emitted. - a helium-4 nucleus, the so-called alpha particle (alpha decay), is emitted - Z decreases by 2, A decreases by 4;
Beta decay (and electron capture) is the transformation of one of the neutrons into a proton, or vice versa, and is a manifestation of the weak force, which “recharges” one of the quarks of the nucleon. An antineutrino is always formed together with an electron, carrying away part of the energy, while the energy between them is redistributed randomly. Because of this, the energy spectrum of beta radiation is continuous.
And alpha decay occurs simply because it is energetically more favorable for any nucleus heavier than iron to “lose weight.” But while this benefit is no more than a few MeV, the energy barrier for removing an alpha particle or any other fragment from the nucleus is too high. And when the energy gain is large enough (but still less than the binding energy), tunneling of the alpha particle outside the nucleus becomes possible. In addition to the alpha particle, in extremely rare cases a neutron or proton, or a nucleus heavier than the alpha particle, can be emitted from the nucleus. And finally, the nucleus can break apart into several nuclei, emitting several neutrons. This is a spontaneous fission that only heavy nuclei, starting with thorium and uranium, are capable of. After the act of decay, excess energy may remain in the core and this “heated” core must somehow get rid of it. To do this, it emits one or more gamma rays. Sometimes the phenomenon of internal conversion also occurs: energy is not emitted in the form of photons, but is transferred to electrons that fly out of the atom. Unlike beta rays, conversion electrons have a monoenergetic (line) spectrum.
In some cases, a nucleus with excess energy can exist for quite a long time, sometimes even hundreds of years. It does not differ from the same “ordinary” nucleus - neither in charge nor in mass, that is, it is the same chemical element and the same isotope. But the isomers
- different.
Most often, the lifetime of metastable isomers does not exceed hours, and only a few of them have years. There is only one nucleus for which only the isomeric state is stable: tantalum-180. In the ground state it is beta-active and short-lived (half-life 8 hours), and its isomer tantalum-180m, it would seem, should either transition to the ground state with the emission of a gamma quantum with an energy of 75 keV, or undergo beta decay, but neither , no one has ever observed any other: this isomer, unlike the short-lived ground state, is stable
.
The decay of a nuclear isomer is the only example of radioactive decay accompanied exclusively by gamma radiation
.
In all other cases, gamma radiation always exists exclusively together
with alpha or beta radiation. We talked about isotopes and isomers. There is one more “iso” left - these are isobars. Nuclei with different nuclear charges and the same mass. Stable isobars usually have charges that differ by two units, and there is almost always a radioactive isotope between them. The existence of two stable isobars in adjacent cells of the periodic table is unlikely - this rule is called the Shchukarev-Mattauch rule. There are only two known exceptions to this: antimony and tellurium-123 and hafnium-180 and the aforementioned tantalum-180m.
Cosmic rays and other non-radioactive sources of radiation
In addition to radioactive substances, some other processes and phenomena, both natural and generated by the human mind, also lead to the appearance of radiation with similar properties.
You probably know about cosmic radiation. Cosmic rays fill the entire Universe, they represent protons and heavier nuclei, electrons and gamma rays with extremely high energies. The maximum energy recorded for cosmic particles reaches zepta
electronvolt! This is eV. It is impossible to say unambiguously what the sources of such high-energy particles are, but particles and gamma rays with moderate energies - from kilo- to gigaelectronvolts - are generated by stars, including our Sun.
This is the so-called primary cosmic radiation. It can only be encountered by entering low-Earth orbit or at least rising several tens of kilometers. Despite their high energy, these particles do not reach the surface. Each of these particles, flying into the atmosphere, causes a cascade of nuclear reactions, leading to the formation of many particles - mainly muons - which then reach the Earth. By the way, they fly solely thanks to relativistic time dilation: the muon’s lifetime is two microseconds; without it, the muon would be able to fly only a little over half a kilometer. And one more interesting fact related to cosmic muons: they are negatively charged, but primary cosmic rays are positively charged, since they consist mainly of protons. This is why the Earth has a negative charge and the ionosphere has a positive charge. At the surface of the Earth, on average, one muon flies through every square centimeter per minute. About a third of the natural background—about 3.5 μR/h—is due to them. And at the altitude at which passenger planes fly, cosmic rays create a dose rate of several microsieverts per hour, already presenting a certain danger to the health of pilots.
In addition to muons, secondary cosmic rays also contain electrons and neutrons. The latter play an important role in the formation of so-called cosmogenic radionuclides.
Secondary cosmic rays have a very high penetrating ability. To protect yourself from them, you have to go into deep basements and mines. Of course, you have to protect yourself from them not because they are harmful to health, but because they interfere with detecting rare and weak events in nuclear physics experiments, measuring small activities of radionuclides, etc. But they also have benefits: with their help, it is possible to “shine through” geological structures and large buildings (such as the Egyptian pyramids).
By the way, the earth's atmosphere is equivalent to about a meter of lead for cosmic rays. It’s not just the atmosphere that protects the Earth and all of us from cosmic rays—it also has a magnetic field that deflects charged particles. But the protective properties of the atmosphere should not be underestimated. During geomagnetic inversions, the Earth’s magnetic shield may practically disappear for a certain time, but contrary to the horror stories of alarmists, this will not lead to the cessation of life on Earth, and the level of radiation at the surface will increase only 2-3 times.
Particularly high-energy particles arriving from space cause a shower of particles to form that covers a large area, causing many particles to be detected simultaneously on detectors separated over considerable distances. These are the so-called widespread atmospheric showers. Their registration with the help of many spaced apart detectors makes it possible to determine the energy of the primary particle, and it is in this way that the energies of the most high-energy particles of cosmic rays are determined. In addition, such a particle causes a powerful flash of Cherenkov radiation in the air, and sources of short-term flashes of gamma radiation and high-energy electrons are lightning and other atmospheric discharges.
And the work of human hands are numerous devices that generate streams of high-energy particles and quanta, not necessarily intentionally. There are X-ray tubes and various types of accelerators specifically for this purpose - from small ones that fit almost in the palm of your hand, to the monster LHC, which occupies the territory of several countries. And the sources, as they say in the dry language of official papers, of unused X-ray radiation are any electric vacuum devices. But it can usually come out at a voltage at the anode of tens of kilovolts. Thus, high-voltage kenotrons, pulsed modulation lamps and microwave traveling wave lamps, klystrons, etc. become sources of X-rays. in radar stations. And also - in the hands of various lovers of home experiments.
You can often hear that the source of X-ray radiation is the kinescope of a TV or monitor. It can, but it usually isn't. The fact is that the glass of the kinescope is quite thick, and x-ray radiation at an anode voltage of 15-25 kV is too soft to pass through such glass. Here are the kinescopes of projection televisions, which operated at voltages up to 50 kV and had small sizes and thin walls of the bulb, “X-rayed” just like that. And among TVs, the ones that “distinguished themselves” were ULPTsT with their anode voltage stabilization circuit. In this circuit, a GP-5 lamp was used, operating at an anode voltage equal to the voltage on the second anode (that is, 25 kV), a noticeable anode current flowed through it, and the walls of this lamp were thin. As a result, it “glowed” brightly in the X-ray range. By placing a sheet of photo paper wrapped in black paper on such a TV, you could get a clear picture of its insides - especially if the protective casing was removed from the lamp.
But we will return to radioactivity.
Uranium and thorium and their daughters
Uranium and thorium became the first radioactive elements known to man.
It was in uranium ore that Henri Becquerel discovered new penetrating radiation similar to X-rays, and it was from it that Marie Skłodowska-Curie extracted the first grains of radium and polonium. These elements are a kind of “islands of stability” in the middle of a sea of elements whose lifespan is too short compared to the lifetime of the Earth. They remain from the times when they were formed in the depths of a supernova, during the explosion of which the gas and dust were formed, from which our Solar system was later formed. And they are located in the midst of elements whose half-lives are measured in minutes, hours, years, millennia... So, by changing the cell in the periodic table to the one next to the right (during beta decay) or to the one to the left, this element turns into even more unstable and a radioactive element that decays again - And so on until the chain of decays finally leads to a stable element - lead or bismuth.
In this regard, in discussions on various forums of radioactive artifacts such as Japanese lenses or uranium glass, as well as stories of depleted uranium in weapons and aircraft, one can often hear a misconception: that uranium and thorium are alpha emitters and therefore their radioactivity can neglect if they do not enter the body. Yes, uranium-238 and thorium-232 undergo alpha decay, which is not accompanied by gamma radiation. However, subsequent members of the uranium-238 series, the decays of which quickly follow one another up to the long-lived uranium-234, are beta active, and protactinium-234m produces intense gamma radiation.
In addition, in natural uranium, in addition to the 238th isotope, there are always 235th and 234th isotopes. The specific activity of the former in natural uranium is 21 times lower than, however, it has intense gamma radiation, like uranium-234, the activity of which is almost always equal to the activity of uranium-238, since it is in secular equilibrium
. Therefore, a piece of uranium-238 “glows” quite decently and illuminates the photographic film on which it lies in about an hour. With thorium, the story is approximately the same, with the only difference being that freshly isolated thorium-232 is indeed an almost pure alpha emitter, and, for example, the thorium glass of Japanese lenses at the time of their manufacture did not pose a particular radiation hazard. But as equilibrium is restored in it, over the course of 10-15 years, the intensity of beta and gamma radiation of thorium increases significantly, which is due to the accumulation of radium-228 and subsequent members of the series in it - up to the final “salute” of thallium-208, which gives a very hard gamma radiation with an energy of 2.6 MeV. This line is usually the last in gamma spectra; behind it there is simply nothing but cosmic radiation.
The most famous “daughter” of uranium-238 is, of course, radium-226, the same one that was discovered by the Curies and the extraction of which Mayakovsky compared his work with:
You exhaust a single word for the sake of Thousands of tons of verbal ore...
But there is almost no radium in fresh uranium.
Before that, there are still 245 thousand years to wait for the decay of uranium-234 and then 75 thousand years for thorium-230 with the beautiful name “ionium”. But in uranium ore, radium is in equilibrium with uranium and its activity is equal to its, uranium, activity. Therefore, uranium ore is much more radioactive than uranium itself. This is why fresh uranium is not a source of radon-222 (minus one more myth about uranium glass).
Thorium also has its own radium in the series - two hundred and twenty-eighth. Since equilibrium in the thorium series is established quickly, radium-228, and with it radon-220, does not keep itself waiting.
A few words about radon
Radon is an inert gas.
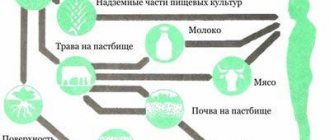
In this regard, it would seem that it should not have a high degree of radiotoxicity, since it is practically not absorbed and does not accumulate. This is what they thought for a long time, and even when a lot was known about the dangers of radiation, radon baths were the most popular method of treatment. But the fact is that radon (uranium is 222, thorium is 220), standing in the middle of the radioactive series, quickly turns into one of the radioactive isotopes of lead (214 for radon and 212 for thoron), which settles in the lungs and remains there forever. Or rather, until it falls apart. And already it (and subsequent members of the series - in the uranium series this is, for example, polonium-210) effectively and efficiently irradiates the lungs. It is radon and its decay products that make the main contribution to the annual radiation dose.
By the way, these radioactive decay products of radon constantly fall on our heads. And if you measure the background radiation on the street during heavy rain, it turns out that it has increased - sometimes even 2-3 times. This is not at all “Chernobyl rain” or the consequences of Fukushima, these are just radon decay products from a kilometer layer of the atmosphere that have collected on the surface of the earth. Then these lead and bismuth-214 will turn into relatively long-lived (22 years) lead-210, which can be used to determine how much time has passed since the layer of sediment at the bottom of the sea or other body of water was covered with new layers.
They are also readily consumed by lichens, such as moss, which deer then feed on. The concentration of daughter products of radon decay in lichens is many times higher than their initial content in rainwater and soil. The content of lead-210 in reindeer moss reaches 500 Bq/kg, which leads to a high content of this nuclide (and therefore polonium-210) in the meat of reindeer - and in the bones of representatives of the peoples of the Far North who rely on this meat (as well as fish, in which also has a high content of lead-210) they feed on. The result is a 35 times higher annual dose than a resident of, for example, Moscow.
About potassium, bananas and other oranges
In addition to uranium and thorium with their “daughters,” sources of natural radioactivity are a number of elements that, in addition to stable ones, also have radioactive natural isotopes.
Among them are isotopes formed during the reign of King Pea before the birth of the Solar System. Their half-lives, like those of uranium and thorium, exceed the lifetime of the solar system, or even the universe. Others have relatively short half-lives, preventing them from surviving ancient times. They could not be formed during the decay of other radioactive isotopes, which means that somewhere there must be another source of their appearance. These are cosmic rays. High-speed protons, crashing into the nuclei of atoms, both themselves cause nuclear reactions and lead to the birth of neutrons and high-energy gamma quanta, which cause new nuclear reactions. As a result, each of the cosmic protons flying into the atmosphere leads to the formation of not only a bunch of muons and electrons, but also to the formation of many unstable nuclei - cosmogenic radionuclides. Due to the fact that they are constantly being formed, they are always present in the atmosphere, despite their relatively short (from seconds to thousands of years) lifetime. Perhaps the most important of the cosmogenic radionuclides is carbon-14, formed from nitrogen under the influence of cosmic rays. Other examples are beryllium-7, which, together with the decay products of radon, can be easily detected in rainwater by its characteristic gamma radiation, tritium.
Some cosmogenic radionuclides were not formed in the Earth's atmosphere under the influence of cosmic rays, but arrived with these cosmic rays. These are chlorine-36 and beryllium-10. Cosmogenic radionuclides are important tracers for studying various natural processes of matter transfer, radioactive “clocks” for dating (everyone knows about the radiocarbon method), but their role in creating the natural radiation background is small - in this, no one except radon can compete with potassium - 40. Their (mainly carbon-14) activity in the human body is only slightly less than the activity of potassium-40, but the latter has a decay energy of one and a half MeV, while carbon-14 has a decay energy of 156 keV. Accordingly, the dose from it is an order of magnitude lower - only about 15 μSv/year.
The peculiarity of potassium is that it is the most important vital element for almost any form of life. And at the same time, potassium is inseparable from radioactive potassium-40, which causes its very noticeable radioactivity. The activity of a gram of natural potassium is 31 Bq/g, and the activity of potassium in the human body is approximately 60 Bq/kg. This activity creates an annual dose of 170 µSv/year - somewhere a little less than one tenth of the total radiation dose.
Bananas are known to be rich in potassium, and therefore its radioactive isotope. In fact, there are a lot of things rich in potassium - dried apricots, dates, nuts, and in general bananas are not the leader among them, but still they have relatively a lot of potassium. The average banana contains about half a gram of potassium, which equates to 15-16 becquerels of potassium. This activity, as well as the magnitude of the contribution to the radiation dose caused by consuming one banana (estimated to be 0.1 µSv) at the time of the Three Mile Island accident, was jokingly nicknamed the "banana equivalent".
In fact, the “banana equivalent” in dose terms is practically zero. The fact is that the concentration of potassium in the body is a fairly constant thing. The body perceives any serious deviation in the concentration of potassium in tissues very painfully and carefully maintains this concentration within narrow limits. If a lot of potassium enters the body, a lot of potassium is excreted by the kidneys. Low potassium - the kidneys will conserve potassium with all their might. But its content in the body will remain unchanged. So eating a banana will not change the amount of potassium in the body, and therefore will not create an additional dose of radiation.
There is also rubidium-87. It also behaves in the body like potassium, but due to its rarity, its contribution to the dose is small - something in the region of 6 μSv/year.
Works of human hands
From the discovery of radioactivity until 1934, scientists dealt only with those radioactive elements that exist in nature.
In 1934, Frederic and Irene Joliot-Curie, studying the formation of free neutrons under the influence of a flux of alpha particles, discovered that after the irradiation stopped, the aluminum target continued to emit certain particles (which later turned out to be positrons), the flux of which quickly faded. This is how the artificial synthesis of a radioactive isotope was carried out for the first time: The formation of radioactive phosphorus was proven chemically: when aluminum that became radioactive was dissolved in hydrochloric acid, all the activity went into the released gas in the form of hydrogen phosphide. Then the Joliot-Curie spouses showed the formation of other artificial radioactive isotopes: radioactive nitrogen was obtained by irradiating boron with alpha particles, and aluminum was obtained by irradiating magnesium. The alchemists' dream of transforming some elements into others has come true. More productive was the use of recently created charged particle accelerators, with the help of which it was possible to synthesize not only many radioactive isotopes of known elements, but also those elements that did not exist in nature. The first of these was technetium, discovered in 1937 by Emilio Segra, whose name has since indicated its artificial origin. Then there were francium, astatine, then the first transuranic elements - neptunium, plutonium... Finally, perhaps the most powerful source of new artificial isotopes was discovered: nuclear fission.
As I said above, for heavy nuclei, the integral existence of an entire nucleus is less energetically beneficial than its destruction. However, the nucleus remains intact, since there is a significant energy barrier between the “whole nucleus” and “separate fragments” states. The probability of spontaneously overcoming such a barrier even for the heaviest nuclei - uranium, thorium, transuranium elements - is insignificant. It is much greater if the separated fragment is an alpha particle, which is what determines the alpha activity of such nuclei. But there remains a very small probability that the nucleus will disintegrate into several approximately identical “pieces”, which will immediately fly apart under the influence of electrostatic repulsion. But the probability of nuclear fission increases sharply if the nucleus is “heated” or excited by some particle from the outside. The easiest way to do this is with a neutron: it does not need to overcome the Coulomb barrier. The excited nucleus becomes deformed and then ruptures. It is important that during fission, not only “fragments” are usually formed, but also free neutrons, which are also capable of causing fission in other nuclei. This process underlies all nuclear energy of our time, and it also produces a huge amount of a wide variety of radioactive isotopes: nuclear “fragments” can be almost anything, and whether we can detect and isolate them or not is determined only by their lifetime. And a powerful stream of neutrons generated during an intense nuclear reaction (especially during a nuclear explosion) is capable of generating very heavy transuranium elements. Einsteinium and fermium became such “children of a nuclear explosion.” And lighter plutonium, americium, curium and californium are produced in reactors in quite industrial quantities.
Reprocessing of irradiated nuclear fuel and neutron irradiation of various elements in reactors have become an effective and cheap source of almost any radioactive isotopes, making it possible to obtain them in any quantities - from small control sources for calibrating pocket dosimeters that come with them and do not pose a serious danger, to those in the beam from which even bacteria almost instantly die, and the air glows like a light bulb.
And then, draining the gasoline and starting the reactor...
A radioactive isotope as a radiation source has one property, which is both an advantage and a disadvantage.
It “works” on its own, not depending on anything. You cannot “turn off” a radioactive source—you can only hide it behind a thick layer of lead. But the fission reaction can (and should) be controlled. The condition for a self-sustaining fission reaction to occur is that the number of neutrons that are produced during fission events is sufficient to replenish both those neutrons that are spent on fission itself, and those that left the core without causing fission: were absorbed or captured, or simply flew beyond its borders. This is a condition of criticality. More neutrons are produced than necessary - the reaction accelerates, exponentially, like an avalanche, increasing its intensity. There are not enough neutrons - the reaction dies out.
Nuclear reactors are usually considered primarily as sources of neutrons. Around such a research reactor (or several) an entire scientific center is usually built, in which a variety of research and experiments are carried out, which require an intense neutron flux. These are studies of crystal structure using neutron diffraction, various methods of chemical analysis based on the transformation of stable elements into radioactive isotopes (neutron activation analysis), the study of the effect of radiation on matter, including biomolecules and living organisms in general, and much more.
One variant of such a reactor is a pulsed nuclear reactor. This is almost like an atomic bomb in the minds of some popularizers of nuclear physics: “if we take two pieces of uranium and put them together, we will get a crater half a mile in diameter.” This is what happens in a pulsed reactor: a critical mass is formed momentarily when one piece of uranium quickly flies past another. The resulting neutron burst can be a thousand times more intense than the neutron flux from a conventional power or research reactor.
A nuclear reactor is a good source of neutrons, but it is stationary, expensive, bulky and dangerous. In the average laboratory or in the field, either californium-252, which generates neutrons through spontaneous fission, or sources based on the reactions of alpha particles with beryllium, boron or aluminum are used to produce a neutron flux. However, such sources are low-intensity and inevitably produce gamma radiation along with neutrons. There is an alternative to such sources in the form of a so-called neutron tube.
In fact, this is also a reactor, only thermonuclear
: A nuclear fusion reaction takes place in a neutron tube. True, much more energy is spent on its implementation than is released, but it produces a neutron flux. And most importantly, a switched-off neutron tube is practically safe (with the exception of some activation of its structural elements and a certain amount of tritium inside the tube) and in this sense is similar to an X-ray tube. Nuclear fusion occurs on a tritium target under the influence of deuterium nuclei - deuterons, accelerated by a gas discharge in deuterium.
Afterword
Ionizing radiation is not a new phenomenon. Contrary to popular beliefs (I have already written about some myths on this topic in previous articles), the share of anthropogenic radiation sources in the radiation dose of the vast majority of people is very small. However, it is anthropogenic sources that pose the greatest danger of acute
radiation injury. Natural radiation in terrestrial conditions almost never directly threatens life - the only exception is work on the development of some of the richest uranium deposits. But artificial sources have already killed many people. These include physicists who worked with uranium and plutonium and were caught in SCR flares, and victims of the bombings of Hiroshima and Nagasaki, and victims of Chernobyl and other lesser-known radiation accidents. There have also been cases where people were killed by a lost or stolen radiation source, or when people unknowingly found themselves in an area of intense radiation and acquired lethal doses within seconds.
I will talk about this - or rather, about radiation safety - in the next article.
All articles in the series
Radiation: Everyday life of a radiochemical laboratory Radiation: units of measurement Radiation: risks, safety, protection
Properties of alpha rays
Alpha rays are the result of a magnetic field affecting a heavy radioactive chemical element. During an explosion, the source of radionuclides is the remnants of the atomic charge of uranium or plutonium, which did not explode. The energy range of alpha rays ranges from 2-9 MeV, depending on what radioactive element was used to produce them. For example, uranium emits alpha rays with an energy of about 4.5 MeV. Moreover, their initial speed is approximately fifteen thousand kilometers per second. As the rays move through the medium, the speed of the alpha particles decreases and over a certain segment becomes equal to the speed of the molecules of this substance. Once slowed down, positive alpha particles attract an electron and form a helium atom.
All the energy of alpha rays is aimed at ionizing the atom. Alpha radiation is recognized as the most ionizing among radioactive ones. Moving one centimeter in the air, particles create about 30 thousand pairs of ions. At the same time, the ionization is not the same at different intervals of the alpha particle's path. The dynamics of growth of the ionizing ability is observed not at the beginning of the travel path, but closer to its end. The highest rates are observed precisely at the end of the path, since the radiation encounters the largest number of atoms, covering the last centimeters.
It is precisely because of the high ionizing ability that the speed of the alpha particle is quite low, and the path length does not exceed 11 cm in the air. In solids, the path length of alpha radiation does not exceed a hundredth of a millimeter. Radionuclides of uranium or plutonium are practically unable to move through the tissues of the human body. An ordinary piece of paper or clothing becomes an insurmountable obstacle for them.
Permissible exposure limits
The standard of ionizing radiation in Russia is regulated by the “Radiation Safety Standards” and the “Basic Sanitary Rules for Working with Radioactive Substances and Other Sources of Ionizing Radiation.” According to these documents, exposure limits are developed for the following categories:
1. "A". This includes employees who work with a radiation source on a permanent basis or temporarily. The permissible limit is calculated as an individual equivalent dose of external and internal radiation per year. This is the so-called maximum permissible dose.
2. "B". The category includes the portion of the population that may be exposed to radiation sources because they live or work near them. In this case, the permissible dose per year is also calculated, at which health problems will not occur for 70 years.
3. "B". This type includes the population of a region, region or country exposed to radiation. Limitation of exposure occurs through the introduction of standards and control of radioactivity of objects in the environment, harmful emissions from nuclear power plants, taking into account dose limits for the previous categories. The impact of radiation on the population is not subject to regulation, since exposure levels are very low. In cases of radiation accidents in the regions, all necessary safety measures are applied.
Characteristics of alpha radiation
Rays of this type are alpha particles with a mass of 4.0015 atomic units. The magnetic moment and spin are zero, and the particle charge is double the elementary charge. The energy of alpha rays is in the range of 4-9 MeV. Ionizing alpha radiation occurs when an atom loses its electron and becomes an ion. The electron is knocked out due to the large weight of alpha particles, which are almost seven thousand times larger than it. As the particles pass through an atom and break off each negatively charged element, they lose their energy and speed. The ability to ionize matter is lost when all the energy is spent and the alpha particle is converted into a helium atom.
How can you protect yourself from alpha radiation?
Alpha radiation is the movement of highly ionized particles that cannot penetrate beyond the upper layers of the epidermis.
The maximum that alpha rays can cause is minor burns and irritation. Based on the results of many studies, it was found that external exposure is not dangerous. However, penetrating into the body through food, water or through damaged areas of the skin, alpha rays can cause serious intoxication. Strong ionization and the presence of free hydrogen and oxygen in alpha radiation lead to serious malfunctions and pathologies. The external flow of alpha rays is considered absolutely harmless to humans and does not require special protection. Plain paper or a thin aluminum sheet will create a reliable barrier. And even this is not required, since clothing will completely block the radiation and prevent it from reaching the skin.
It is recommended to move 20 cm away from the source of alpha rays and such a precaution will be quite sufficient.
As for internal radiation, you need to carefully take care of your own safety. The equipment of a person located in a mass destruction zone should include the following personal protective equipment:
- clothing and footwear made of dense materials: overalls with hoods, sleeves, gloves, special boots;
- helmet and visor made of plexiglass, the best uniform would be a gas mask;
- alpha rays penetrate damaged skin and wounds, so it must be protected with special pastes, emulsions or creams.
In addition, there are recommendations regarding the removal of alpha particle breakdown products from the body by consuming certain foods. Among them are citrus fruits, cabbage, legumes, fish and other products that contain vitamins B and C. As for traditional methods, Jerusalem artichoke promotes the release of radionuclides from the body.
The special properties of alpha particles, in particular their low penetrating ability, do not allow radiation to be detected by conventional dosimeters. For this, a Geiger counter is used, which notifies of danger with a characteristic click, which allows you to quickly protect yourself and protect yourself from the penetration of alpha rays.
Security measures
Alpha radiation protection is not a problem. Radiation rays are completely blocked by a thick sheet of paper and even human clothing. The danger arises only from internal exposure. To avoid it, personal protective equipment is used. These include overalls (overalls, moleskin helmets), plastic aprons, oversleeves, rubber gloves, and special shoes. To protect the eyes, plexiglass shields are used, dermatological products (pastes, ointments, creams), and respirators are also used. Enterprises are resorting to collective protection measures. As for protection from radon gas, which can accumulate in basements and bathrooms, in this case it is necessary to frequently ventilate the premises and insulate the basements from the inside.
The characteristics of alpha radiation lead us to the conclusion that this type has a low throughput and does not require serious protective measures during external exposure. These radioactive particles cause great harm when they penetrate into the body. Elements of this type extend over minimal distances. Alpha, beta, and gamma radiation differ from each other in their properties, penetrating ability, and impact on the environment.
Every substance on earth is radioactive to one degree or another. Alpha radiation is a stream of heavy particles with a positive charge, consisting of protons and neutrons.
Scientists are constantly studying the possibilities of using radiation nuclides in medicine and successfully treating cancer diseases using their help. However, alpha particles should be handled with extreme caution as they have very high levels of biological activity. What does the term “alpha radiation” mean, how was it discovered and is it dangerous to humans?
Features of α-rays in different environments
In addition to the need to know what alpha radiation is to protect yourself from its influence, you need to understand its features.
The starting speed of such particles varies between 14-20 thousand km/s. Compared to beta particles, they are considered more massive. The difference is more than 7300 times. Because of this, the ionizing ability of the rays is considered high.
The average ion vapor creation rate here is 200,000 times. To do this, basic conditions must be met: free movement in the air, an ambient temperature of 15 degrees and normal atmospheric pressure.
But the “viability” of these particles is quite limited. This is due to the fact that ionization requires numerous energy costs. Once the particles begin to consistently decelerate, their ability to ionize increases significantly.
The free path of alpha-gamma particles through the air is no more than 11 cm in a favorable environment. But liquid and solid media are not favorable for the penetration of rays. Here they cannot move even a millimeter.
The effect of alpha radiation on humans
Significant ionization leads to the fact that the powerful flow of energy that comes from the source is very quickly reduced to zero.
Due to such a lightning-fast loss of energy resource, the penetrating power of an alpha particle is hundredths of a millimeter. Radiation is not able to pass even through dead skin cells, so it poses virtually no danger to humans when exposed externally. If an accelerator was used to produce alpha radiation, then its influence will no longer be so harmless. Alpha particles instantly decay into radioactive nuclides, which are dangerous to human health.
Ingesting the smallest dose of radiation, which can enter the body through the respiratory system or digestive tract, can result in radiation exposure sufficient to cause radiation sickness.
That is, when exposed externally, alpha rays can harm the body only if the human body is covered with open wounds. Once in the body, alpha particles cause cells to divide at a faster rate, irradiating them, which leads to changes in genetic information, mutations and the formation of cancerous tumors. When alpha radiation penetrates into the body, it can lead to radiation sickness - a fatal outcome is inevitable.
Penetration ability
Since an alpha particle has a significant mass (compared to the mass of an electron), as well as an electric charge, which in magnitude exceeds the charge of an electron by 2 times, its penetrating ability, that is, the ability to pass through a layer of matter, is insignificant.
During its movement, the alpha particle experiences collisions with atoms, transferring to them a significant amount of energy, which leads to the ionization of atoms, that is, to the separation of electrons from them. For example, passing just 5 cm in air, an alpha particle experiences a huge number of collisions and almost completely loses its kinetic energy.
Any solid substance easily traps an alpha particle. Thus, it cannot pass through a layer of several sheets of paper, and an aluminum plate with a thickness of only 0.1 mm delays a flow of alpha particles of any intensity. Let us note again that although the penetrating ability of this particle is small, it very strongly ionizes any substance through which it moves.