Highly radioactive background (smog) is a product of the decay of atoms with subsequent changes in their nuclei. Elements with this ability are considered highly radioactive. Each compound is endowed with a certain ability to penetrate the body and harm it. They are natural and artificial. Gamma radiation has the strongest penetrating ability - its particles are able to pass through the human body and are considered very dangerous to human health.
People working with them must wear protective clothing, since their impact on health can be very strong - it depends on the type of radiation.
Types and characteristics of radiation
There are several types of radiation. People in their line of work have to deal with it - some every day, some from time to time.
Alpha radiation
Helium particles carry a negative charge and are formed during the decay of heavy compounds of natural origin - thorium, radium, and other substances of this group. Streams with alpha particles cannot penetrate solid surfaces and liquids. To protect against them, a person just needs to be dressed.
Beta rays
This type of radiation has more power compared to the first type. For protection, a person will need a dense screen. The decay product of several radioactive elements is a flux of positrons. They are separated from electrons only by charge - they carry a positive charge. If they are exposed to a magnetic field, they deflect and move in the opposite direction.
Gamma radiation
It is formed during the decay of nuclei in many radioactive compounds. The radiation has a high penetrating ability. Characterized by hard electromagnetic waves. To protect against their effects, you will need screens made of metals that can well protect a person from penetration. For example, made of lead, concrete or water.
X-ray radiation
These rays have great penetrating power. Can be formed in X-ray tubes, electronic installations such as betatrons and the like. The nature of the action of these radioactive streams is very strong, which suggests that the X-ray beam is endowed with the ability of strong penetration, and therefore dangerous.
In many ways similar to the above, it differs only in the length and origin of the rays. The X-ray flux has a longer wavelength with a low radiation frequency.
Ionization here is carried out mainly by knocking out electrons. And due to the consumption of its own energy, it is produced in small quantities.
Undoubtedly, the rays of this radiation, especially hard ones, have the greatest penetrating ability.
Penetrating power of radiation
Alpha particles have the smallest penetrating ability: the range in air is several centimeters, in biological tissue - fractions of a millimeter. Therefore, thick clothing provides the necessary and sufficient degree of protection from external alpha radiation. Beta particles (electron flow) have greater penetrating power: their range in the air is several meters, in biological tissue - up to several centimeters. Therefore, when working with sources of hard beta radiation, there is a need to use additional protection (protective screens, containers). Finally, gamma radiation has the greatest penetrating ability: electromagnetic waves are able to pass through the body. Sources of powerful gamma radiation require heavier protection: lead screens, thick-walled concrete structures.
What type of radiation is most dangerous for people?
The hardest quanta are X-ray waves and gamma radiation. They have the shortest waves, therefore, they bring more treachery and danger to the human body. Their insidiousness is explained by the fact that a person does not feel their influence, but clearly feels the consequences. Even at low doses of radiation, irreversible processes and mutations occur in the body.
The transmission of information within a person is electromagnetic in nature. If a powerful radiation beam penetrates the body, this process is disrupted. A person initially feels a slight malaise, and later pathological disorders - hypertension, arrhythmia, hormonal disorders and others.
Alpha particles have the lowest penetration ability, so they are considered the safest, so to speak, for humans. Beta radiation is much more powerful and its penetration into the body is more dangerous. Radiation from gamma particles and X-rays has the greatest penetrating power. They are able to pass right through a person, it is much harder to protect against them, and only a concrete structure or a lead screen can stop them.
Types of radiation
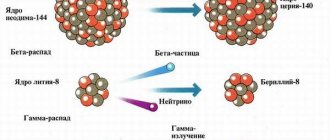
There are three main types of radiation emitted by radioactive nuclei.
- alpha radiation
- It is a stream of alpha particles consisting of two protons and two neutrons (actually, these are the nuclei of helium atoms) formed as a result of the alpha decay of heavy nuclei.
- beta radiation
- This is a stream of electrons or positrons (beta particles) formed as a result of the beta decay of radioactive nuclei.
- gamma radiation
- Gamma radiation accompanies alpha or beta decay and is a stream of gamma quanta, being, in fact, electromagnetic radiation - that is, it has a wave nature similar to the nature of light. The difference is that gamma quanta have much higher energy than light quanta and therefore have greater penetrating power.
How is electromagnetic smog determined in a residential apartment?
Every comfortable apartment has a certain level of radioactive waves. They come from household electronic appliances and devices. Electromagnetic smog is determined by a special device - a dosimeter. It’s good when it is present, but if it is not, then they can be identified in another way.
To do this, you need to turn on all electrical devices and use a regular radio to check the radiation level of each of them. If interference occurs in it, squeaking, extraneous noise and crackling are heard, then there is a source of smog nearby. And the more tangible they are, the more powerful and stronger the electromagnetic radiation emanates from it. The source of smog can be the walls of the apartment. Any actions taken by residents to protect their own bodies from their effects are a guarantee of health.
Sources of radiation
In general, it is important to understand that radionuclides are not the only sources of radiation. In particular, when undergoing an annual fluorographic examination or having a computed tomography scan, we are exposed to x-ray radiation, which (like gamma radiation) is a stream of quanta. This means that the two types of radiation, having different origins, are equally classified as penetrating radiation. In other words, although the X-ray tube does not use radionuclides, it also produces ionizing radiation.
Half life
The number of decays per second in a radioactive source is called activity
.
The unit of activity is
the becquerel (Bq, Bq): 1 Bq is equal to one decay per second.
The time during which on average half of all radionuclides of a given type in any radioactive source decay is called the half-life. The decrease in the concentration of radionuclides in the body by half is called the half-life. For example, on the territory of Ukraine, as a result of the Chernobyl accident, the following radionuclides with half-life and half-life periods fell: carbon 14 - 5730 years and 200 days, respectively; cesium 137, 30 years and 100 days, respectively; strontium 90 – 29 and 20 years, respectively; iodine 131 – 8 and 138 days, respectively. The area becomes safe for living and use after approximately 10 half-lives.
Beta radiation
- emitted: electrons or positrons
- penetration: medium
- irradiation from source: up to 20 m
- radiation speed: 300,000 km/s
- ionization: from 40 to 150 ion pairs per 1 cm of travel
- biological effect of radiation: average
Beta (β) radiation occurs when one element transforms into another, while the processes occur in the very nucleus of the atom of the substance with a change in the properties of protons and neutrons.
With beta radiation, a neutron is transformed into a proton or a proton into a neutron; during this transformation, an electron or positron (electron antiparticle) is emitted, depending on the type of transformation. The speed of the emitted elements approaches the speed of light and is approximately equal to 300,000 km/s. The elements emitted during this process are called beta particles.
Having an initially high radiation speed and small sizes of emitted elements, beta radiation has a higher penetrating ability than alpha radiation, but has hundreds of times less ability to ionize matter compared to alpha radiation.
Beta radiation easily penetrates through clothing and partially through living tissue, but when passing through denser structures of matter, for example, through metal, it begins to interact with it more intensely and loses most of its energy, transferring it to the elements of the substance. A metal sheet of a few millimeters can completely stop beta radiation.
If alpha radiation poses a danger only in direct contact with a radioactive isotope, then beta radiation, depending on its intensity, can already cause significant harm to a living organism at a distance of several tens of meters from the radiation source.
If a radioactive isotope emitting beta radiation enters a living organism, it accumulates in tissues and organs, exerting an energetic effect on them, leading to changes in the structure of the tissue and, over time, causing significant damage.
Some radioactive isotopes with beta radiation have a long decay period, that is, once they enter the body, they will irradiate it for years until they lead to tissue degeneration and, as a result, cancer.
X-ray radiation
- emitted: energy in the form of photons
- penetration: high
- irradiation from source: up to hundreds of meters
- radiation speed: 300,000 km/s
- ionization: from 3 to 5 ion pairs per 1 cm of travel
- biological effect of radiation: low
X-rays are energetic electromagnetic radiation in the form of photons that occur when an electron inside an atom moves from one orbit to another.
X-ray radiation is similar in effect to gamma radiation, but has less penetrating power because it has a longer wavelength.
Having examined the various types of radioactive radiation, it is clear that the concept of radiation includes completely different types of radiation that have different effects on matter and living tissues, from direct bombardment with elementary particles (alpha, beta and neutron radiation) to energy effects in the form of gamma and x-rays cure.
Each of the radiations discussed is dangerous!
Natural radioactive background
The world's population is constantly exposed to natural background radiation. This is cosmic radiation (protons, alpha particles, gamma rays), radiation from natural radioactive substances present in the soil, and radiation from those radioactive substances (also natural) that enter the human body with air, food, and water. The total dose generated by natural radiation varies greatly in different areas of the Earth. In Ukraine it ranges from 70 to 200 mrem/year.
Natural background provides approximately one third of the so-called population dose of the general background. Another third of people receive it during medical diagnostic procedures - x-rays, fluorography, x-rays, etc. The rest of the population dose comes from human stay in modern buildings. Coal-fired thermal power plants also contribute to increased background radiation, since coal contains dispersed radioactive elements. When flying on airplanes, a person also receives a small dose of ionizing radiation. But all these are very small quantities that do not have a harmful effect on human health.
Video:
It just so happened that from the very beginning, nuclear energy was created in deep secrecy and secrecy, including from its own people. She remained in this state for many years. As for educating the population on the basics of nuclear ecology and health protection from ionizing radiation, nuclear scientists practically did not deal with these issues. After all, the less people understand about these matters, the easier it is to “turn them off” or deceive them.
And it is no coincidence that the population of our region, living next to the large atomic research center RIAR, has very little or no understanding of even basic issues related to ionizing radiation.
In order to improve the situation, we decided in this issue of the “Civil Initiative” newsletter to open a nuclear educational program and publish information on at least the basic concepts related to ionizing radiation or, as they say in everyday life, radiation. We had to sort through a lot of relevant material to select the clearest and most simple explanations. In the end, we chose information from the journal “Physics”, taking it as a basis and supplementing it from other sources, including from the appendix to the book “Atomic Mythology” by corresponding member of the Russian Academy of Sciences A.V. Yablokov.
Below are answers to questions that appear in letters from our readers and in conversations with residents of the region.
Question. What is a nuclide, radionuclide, isotope?
Answer. Nuclide
is called an atomic nucleus, characterized, firstly, by a certain nucleon composition (the number of protons and neutrons) and, secondly, by a certain energy state.
Nuclei that have the same nucleonic composition but different energy states are called nuclear isomers
.
Nuclei that retain their nucleonic composition and energy state for an indefinitely long time are called stable; otherwise we are talking about radioactive nuclides, radionuclides
. There can be two or more nuclear isomers, but only one of them is a stable nuclide.
Radionuclides are often called isotopes. This is incorrect: the concept of isotopes
a set of nuclides (both stable and radioactive) is determined that have the same number of protons (and are therefore chemically identical, since these nuclides naturally have the same atomic number and are species of the same element from the periodic table).
Question. What is radioactivity and radiation?
Answer. Radioactivity
there is the property of some radionuclides to change over time their nucleonic composition and (or) energy state with the formation of new nuclides (stable or again radioactive) and the emission of IONIZING RADIATION with greater or lesser PENETRATION.
These radiations are commonly called radiation
.
Question. What is activity?
Answer. Activity
radionuclide source or drug is the number of radioactive transformations in it per unit time.
The unit of activity is the becquerel
(Bq) - the activity of a source in which (on average, in a statistical sense) 1 radioactive transformation occurs in 1 second. In practical radiation measurements the following are often used: kilobecquerel (1 kBq = 10 3 Bq); megabecquerel (1 MBq = 10 6 Bq); gigabecquerel (1 GBq = 10 9 Bq).
the curie, is still often used.
(Ki). 1 Ci corresponds to the activity of 1 g of radium-226 in equilibrium with its daughter decay products. The title and semantic content are echoes of the history of nuclear physics, one of the pages of which was the isolation of radium from uranium ore by Marie and Pierre Curie and the study of its properties.
1 Ci = 3.7*10 10 Bq (37 GBq) is a very large (in everyday terms) activity, therefore in practice they often use: millicurie (1 mCi = 10 -3 Ci); microcurie (1 µCi = 10 -6 Ci); nanocurie (1 nCi = 10 -9 Ci).
Question. Are all radiations ionizing? Which ones are ionizing?
Answer.
No, not all, but only those whose energy is capable of causing ionization. For example, electromagnetic radiation in the range of radio waves or visible light is not ionizing radiation. Nuclear radiation, characterized by significant energy of each individual particle, is a different matter.
To consider processes and phenomena related to nuclear technology and energy, as well as radiation safety and radioecology, the following types of nuclear ionizing radiation are essential:
1. Alpha (a) radiation.
This is the emission of nuclear particles, each of which consists of 2 protons and 2 neutrons (helium nucleus). It occurs during the decay of atomic nuclei heavier than lead (for example, uranium, thorium, radium, plutonium), as well as in many nuclear reactions. The entry of an alpha emitter into the body can cause biological damage to its cells, because The alpha particle carries a large amount of energy and its ionizing ability is very high.
2. Beta (b) radiation.
This is the emission of electrons and positrons moving at very high speeds. It occurs mainly as a result of radioactive decay. The ionizing ability is significantly lower than that of a-radiation. However, beta particles are dangerous when they get on the surface of the body or inside the body.
3. Gamma (g) radiation
- the shortest wavelength electromagnetic radiation of high energy and has the greatest penetrating ability. Accordingly, protection from external gamma radiation poses the greatest challenges.
Question. What is the penetrating power of radiation?
Answer. Penetrating power of radiation
determines the composition and thickness of the material that effectively absorbs it.
a-radiation is the least penetrating. It is effectively absorbed by a layer of air several centimeters thick, a layer of water about 0.1 mm thick, or, for example, a sheet of paper. b-radiation has a significantly greater penetrating ability; to stop it, you need, for example, a layer of aluminum several millimeters thick, and the range of beta particles in biological tissue reaches several centimeters. For g-radiation, all these barriers are almost transparent. To detain it, you need a very thick (tens of centimeters and even meters) layer of a substance with as high an atomic number as possible (for example, lead).
The above is illustrated by the figure. It is easy to see that for a -, b - and g - radiations a simple pattern is observed: the higher the ionizing ability of the radiation, the lower the penetrating ability. This is not at all accidental - when these radiations interact with matter, the main part of the energy is spent on ionization.
Question. What are “exposure dose”, “absorbed dose”, “equivalent dose”, “effective equivalent dose” and what are their units of measurement?
Answer. Exposure dose
- a measure of gamma radiation energy, determined by the ionization of air. Expressed in Roentgens (R) per unit of time: Roentgen per hour (R/h) or micro-Roentgen per hour (µR/h), etc.
1 Roentgen is equal to 1000 milliRoentgens or 1,000,000 microRoentgens.
Absorbed dose
- the amount of energy of any type of ionizing radiation absorbed by a unit mass of the irradiated substance (the main dosimetric quantity). The unit of absorbed dose is 1 Gray (Gy).
Equivalent dose
- absorbed dose for different types of radiation (i.e. multiplied by a coefficient for different types of ionizing radiation), causing the same biological effect (the main dosimetric value for assessing damage to human health from chronic exposure to radiation of arbitrary composition). The coefficient for beta, gamma, and x-ray radiation is 1, for alpha radiation - 20.
According to the SI system, the equivalent dose is measured in Sieverts (abbreviated as Sv). The name of this unit of measurement is given in memory of Sievert, a Swedish radiologist. Previously, we more often used another unit of measurement - the rem (the biological equivalent of an x-ray). 1 Sv is equal to 100 rem.
The derivative of the equivalent dose is the effective equivalent dose
— Sievert per unit of time. For example, milliSievert/year (abbreviated mSv/year), microSievert/year (abbreviated μSv/year).
Question. In what units is radiation pollution measured?
Answer.
Radiation contamination of an area is expressed in Curies per square kilometer or Becquerels per square kilometer. Radioactive contamination of liquids, products and other substances is expressed in Becquerels per liter or kilogram (Bq/l, Bq/kg).
For information: More detailed information can be obtained from our “Center for the Promotion of Civil Initiatives”, where relevant literature is available on these issues.
| Content | > |
Radioactive radiation (or ionizing radiation) is energy that is released by atoms in the form of particles or waves of an electromagnetic nature. Humans are exposed to such exposure through both natural and anthropogenic sources.
The beneficial properties of radiation have made it possible to successfully use it in industry, medicine, scientific experiments and research, agriculture and other fields. However, with the spread of this phenomenon, a threat to human health has arisen. A small dose of radioactive radiation can increase the risk of acquiring serious diseases.
Alpha radiation
- emitted: two protons and two neutrons
- penetration: low
- irradiation from source: up to 10 cm
- radiation speed: 20,000 km/s
- ionization: 30,000 ion pairs per 1 cm of travel
- biological effect of radiation: high
Alpha (α) radiation occurs from the decay of unstable isotopes
elements.
Alpha radiation is the emission of heavy, positively charged alpha particles, which are the nuclei of helium atoms (two neutrons and two protons). Alpha particles are emitted during the decay of more complex nuclei, for example, during the decay of atoms of uranium, radium, and thorium.
Alpha particles have a large mass and are emitted at a relatively low speed of an average of 20 thousand km/s, which is approximately 15 times less than the speed of light. Since alpha particles are very heavy, upon contact with a substance, the particles collide with the molecules of this substance, begin to interact with them, losing their energy, and therefore the penetrating ability of these particles is not great and even a simple sheet of paper can hold them back.
However, alpha particles carry a lot of energy and, when interacting with matter, cause significant ionization. And in the cells of a living organism, in addition to ionization, alpha radiation destroys tissue, leading to various damage to living cells.
Of all types of radiation, alpha radiation has the least penetrating power, but the consequences of irradiation of living tissues with this type of radiation are the most severe and significant compared to other types of radiation.
Exposure to alpha radiation can occur when radioactive elements enter the body, for example through air, water or food, or through cuts or wounds. Once in the body, these radioactive elements are carried through the bloodstream throughout the body, accumulate in tissues and organs, exerting a powerful energetic effect on them. Since some types of radioactive isotopes emitting alpha radiation have a long lifespan, when they enter the body, they can cause serious changes in cells and lead to tissue degeneration and mutations.
Radioactive isotopes are actually not eliminated from the body on their own, so once they get inside the body, they will irradiate the tissues from the inside for many years until they lead to serious changes. The human body is not able to neutralize, process, assimilate or utilize most radioactive isotopes that enter the body.
The effect of radiation on human health
Radioactive radiation, due to its ionizing effect, leads to the formation of free radicals in the human body - chemically active aggressive molecules that cause cell damage and death.
Cells of the gastrointestinal tract, reproductive and hematopoietic systems are especially sensitive to them. Radioactive radiation disrupts their work and causes nausea, vomiting, bowel dysfunction, and fever. By affecting the tissues of the eye, it can lead to radiation cataracts. The consequences of ionizing radiation also include damage such as vascular sclerosis, deterioration of immunity, and damage to the genetic apparatus.
The system of transmission of hereditary data has a fine organization. Free radicals and their derivatives can disrupt the structure of DNA, the carrier of genetic information. This leads to mutations that affect the health of subsequent generations.
The nature of the effects of radioactive radiation on the body is determined by a number of factors:
- type of radiation;
- radiation intensity;
- individual characteristics of the body.
The effects of radioactive radiation may not appear immediately. Sometimes its consequences become noticeable after a significant period of time. Moreover, a large single dose of radiation is more dangerous than long-term exposure to small doses.
The amount of radiation absorbed is characterized by a value called Sievert (Sv).
- Normal background radiation does not exceed 0.2 mSv/h, which corresponds to 20 microroentgens per hour. When X-raying a tooth, a person receives 0.1 mSv.
- The lethal single dose is 6-7 Sv.
Neutron radiation
- emitted: neutrons
- penetration: high
- exposure from source: kilometers
- radiation speed: 40,000 km/s
- ionization: from 3000 to 5000 ion pairs per 1 cm of travel
- biological effect of radiation: high
Neutron radiation is man-made radiation that occurs in various nuclear reactors and during atomic explosions. Also, neutron radiation is emitted by stars in which active thermonuclear reactions occur.
Having no charge, neutron radiation colliding with matter weakly interacts with the elements of atoms at the atomic level, and therefore has high penetrating power. You can stop neutron radiation using materials with a high hydrogen content, for example, a container of water. Also, neutron radiation does not penetrate polyethylene well.
Neutron radiation, when passing through biological tissues, causes serious damage to cells, since it has a significant mass and a higher speed than alpha radiation.
Gamma radiation
- emitted: energy in the form of photons
- penetration: high
- irradiation from source: up to hundreds of meters
- radiation speed: 300,000 km/s
- ionization: from 3 to 5 ion pairs per 1 cm of travel
- biological effect of radiation: low
Gamma (γ) radiation is energetic electromagnetic radiation in the form of photons.
Gamma radiation accompanies the process of decay of atoms of matter and manifests itself in the form of emitted electromagnetic energy in the form of photons, released when the energy state of the atomic nucleus changes. Gamma rays are emitted from the nucleus at the speed of light.
When the radioactive decay of an atom occurs, other substances are formed from one substance. The atom of newly formed substances is in an energetically unstable (excited) state. By influencing each other, neutrons and protons in the nucleus come to a state where the interaction forces are balanced, and excess energy is emitted by the atom in the form of gamma radiation