Home Ι The harm of alcohol
Ethanol is not the only representative of the class of alcohols, the main feature of which is the presence of a hydroxyl group. There are other compounds that differ from each other in the number of carbon atoms. They have similar but not the same properties.
Ethyl alcohol is used in cooking, medicine and is part of alcoholic beverages. Methanol provokes severe poisoning. If first aid measures are not taken promptly, there is a high probability of death from consuming this compound.
The main differences between ethanol and methanol
A specialist in the field of chemistry will confirm that methanol and ethanol belong to the class of organic compounds “alcohols”. Methyl is the first representative of this series. It has only one carbon atom connected to a hydroxyl group. Ethyl alcohol is the next in a series of similar compounds. It already contains two carbon atoms, one of which is bonded to oxygen and hydrogen.
Methanol also has other names: carbinol, wood alcohol. In terms of its physical properties, it is as close as possible to ethanol. It is a colorless and transparent liquid with a characteristic pungent odor. It is for this reason that it is often difficult to differentiate one alcohol from another.
Methanol and ethanol have different effects. Ethyl alcohol has less toxicity, but can provoke a state of intoxication. This determines its widespread use in the production of alcohol, when it comes to fortified drinks with the addition of a chemically synthesized substance. In addition, ethyl alcohol is used in medicine as an excellent disinfectant and serves as the basis for many medicines.
Methanol is essentially technical alcohol. It is indispensable in the chemical and paint and varnish industries, but its use for food purposes is strictly prohibited due to its high toxicity. Even small doses can provoke severe poisoning, leading to disability or death.
Unfortunately, methyl alcohol intoxication is not uncommon. The substance may be present in low-quality cheap alcohol. It is for this reason that you can only purchase proven products with excise stamps. Another cause of poisoning is the ingestion of liquids that are not intended for drinking for the purpose of intoxication. As practice shows, most often these are windshield wipers.
Important! The human body reacts very sharply to the presence of methanol inside. Even if 10 ml is ingested, the first symptoms of poisoning develop. If a person takes more than 30 ml, then disability cannot be avoided. Only 50 ml is considered the critical mark. This almost always guarantees death.
Methanol impurities in alcohol are not so easy to detect. Usually people react only when signs of acute intoxication appear. Doctors report that the narcotic properties of ethanol are higher than those of wood alcohol. For this reason, a person who drinks surrogate alcohol with carbinol will become drunk more slowly.
Surrogate alcohol, its varieties
The risk of poisoning from this toxic liquid is very high, since it is practically no different from ethyl alcohol. Carbinol has the same smell, taste and clarity as other compounds used to make alcoholic beverages. Therefore, it is imperative to know what early symptoms of methyl alcohol poisoning occur in order to take timely measures.
If you have doubts about the quality of the purchased alcohol, it is better to refuse to take it. You can do it differently, for example, check what is contained in this liquid.
To prevent carbinol intoxication, you should drop a little liquid into a spoon and then set it on fire. If there are more green colors in the flame, then this alcohol contains technical alcohols. It must be remembered that its color when ethanol burns is always blue.
In addition, regular potatoes will help detect the methanol content in alcoholic beverages. This vegetable acts as an indicator. With its help, you can understand whether there are toxins in the liquid or not. To test the drink, you need to take some peeled potatoes and place them in a container with liquid.
After some time, when the chemical reaction is completed, the root vegetable may acquire a pale pink tint. With such changes, it is immediately clear that carbinol is definitely present in the alcohol.
Car enthusiasts should use wood alcohol-based products very carefully. It must be remembered that their vapors are also toxic, so it is better not to breathe them. It is advisable to cover your face with a mask and use rubber gloves. If, however, symptoms of methyl alcohol poisoning appear, you should immediately consult a doctor.
You should drink only high-quality and proven alcoholic products. It must be purchased in specialized stores that have a trading license. You shouldn't buy drinks secondhand to save a little money, it can be life-threatening!
There are three types of counterfeit and deadly alcohol:
- true surrogates are alcohol tinctures not for internal use, household chemicals, as well as methyl alcohol itself;
- liquids that do not contain ethyl (edible) alcohol, which include methyl, formic and butyl alcohols;
- counterfeit alcoholic drinks containing methyl.
Industrial alcohol is indistinguishable from ethanol in smell and taste, so recognizing it is not at all easy. In rare cases, it may even be contained in alcohol purchased at a nearby store.
As a rule, methyl alcohol poisoning occurs when consuming counterfeit alcohol products. Less common are suicide attempts using liquids containing methanol. It can also pose a serious risk to a child if the container is stored in an accessible place and is easily opened.
In industrial conditions, intoxication through the respiratory tract is possible due to prolonged inhalation of toxic methanol vapors.
In this regard, basic preventive measures should include:
- Store the substance in a tightly closed container with appropriate labeling. It should be located in a place inaccessible to children;
- Using antifreeze based on ethylene glycol or propylene glycol, which are less toxic compared to methanol;
- Drinking quality alcoholic beverages. You need to purchase them from large retail chains that carefully check the products they receive. If in doubt, the drink can be checked through online resources using the excise stamp number;
- Compliance with industrial safety regulations when working with toxic liquids;
- Use of medical and cosmetic products strictly for their intended purpose. Do not ingest liquids intended for external use only.
Methods for distinguishing methanol from ethyl alcohol are quite complex. They are rarely used in everyday life.
- Dip a hot copper wire into a container with an unknown liquid and notice the aroma that appears. Copper reacts with ethanol to form acetaldehyde, which smells like sour apples. Methanol, on contact with copper wire, releases formaldehyde with a pungent, unpleasant odor.
- Pour the liquid into a metal container and set it on fire. When ethyl alcohol is burned, the flame has a bluish tint; when methanol is ignited, it has a greenish tint.
- Place the container on the fire: methyl alcohol will boil earlier (at 65 °C), and ethyl alcohol will boil a little later and begin to boil at 79 °C.
- Peel small potatoes and cover with alcohol. If after a few hours the liquid becomes cloudy and the potatoes take on a pinkish tint, then you are dealing with methanol. If the color of the vegetable has not changed, but the alcohol remains transparent, then this is ethanol.
Remember that if you have the slightest uncertainty about the quality of alcohol, you should not drink it orally. Even when conducting the described experiments, it can be difficult for people without special education to evaluate their results. To minimize the risk of poisoning, do not drink liquids of questionable origin and use caution when working with chemicals.
We suggest you read What to do if your wife drinks: how to wean your wife from drinking
Today in Russia strict records are kept of the production and sale of alcohol and alcohol-containing products, for which the Unified State Automated Information System has been developed. However, cases of consumption of counterfeit alcohol still occur, leading to death from methyl alcohol poisoning. To protect yourself, you should purchase alcohol only in large stores that have a license to sell alcoholic beverages.
How to distinguish ethanol from methanol
Methanol has chemical properties that are different from ethyl alcohol, so several experiments can be done to determine its presence in alcoholic beverages.
One of the differences between substances in the alcohol class is their ability to burn, and the easiest way to distinguish methanol from ethyl alcohol is to set it on fire. The first burns with a green flame, and the second with a blue flame. This method will help if the methanol content in the drink is high enough.
An indicator of the presence of methanol is regular potatoes. Dip a slice of peeled root vegetable into the liquid. The potatoes will turn pink, a sign that the liquid contains methanol. If it is not there, then there will be no changes in the vegetable or it will turn a little blue.
You can also conduct a formaldehyde test at home. For this you will need copper wire. For the experiment, it is heated up strongly with a lighter and dipped into a suspicious drink. The presence of methanol gives a sharp and unpleasant formaldehyde odor. Ethyl alcohol with this reaction will give a slight aroma reminiscent of apple cider vinegar.
If the household has a heat-resistant thermometer, you can conduct another experiment. Place the suspect drink in a saucepan or bowl and heat on the stove. The boiling point of methyl alcohol is about 65 degrees. Ethanol boils at a temperature of 78-80 degrees.
To avoid accidents, you should buy alcohol and alcohol-containing products only from large retail outlets with a state license.
The effect of methanol on the body
It is important to understand that any substance that penetrates into the body undergoes changes due to synthesized enzymes. But this happens only if the body is able to break down the compound that enters it. Thus, ethyl alcohol is quite successfully processed and excreted in the form of safe compounds, provided that the person does not suffer from alcohol intolerance.
Methyl alcohol enters through the digestive system. From the stomach it penetrates directly into the duodenum. The inner surface of this organ is covered with tiny villi in which capillaries lie. Not only nutrients from food, but also alcohol-containing liquids are distributed through them. It is in this way that methanol is distributed throughout the body.
Many people are interested in how long it takes for wood alcohol poisoning to manifest itself. According to doctors, this happens quite quickly. The first signs of intoxication can be noticed within a few minutes. If you have time to provide first aid, then there is still a chance to save life.
Inside the body, methanol is exposed to the liver enzyme alcohol dehydrogenase. However, if in the case of ethanol, acetaldehyde is formed at the primary stage of oxidation, which provokes a hangover, then the situation here becomes even more dangerous. As a result of transformations, formic aldehyde is synthesized - a substance with the highest toxicity. Its other name is formaldehyde.
This compound will later be oxidized to formic acid, which is also dangerous to internal organs. Methanol breakdown products are eliminated primarily through the kidneys, but the lungs are also involved in this process. Both structures are significantly damaged when wood alcohol metabolites are eliminated.
In addition, methanol can accumulate in tissues, which provokes serious disruptions in the functioning of various body systems. So, the central nervous system reacts quite sharply. The activity of the nervous system is suppressed almost immediately after consuming minimal doses of a dangerous substance. Next, the visual analyzer is affected, and then the cardiovascular system.
How does methyl alcohol work?
The causes of methanol poisoning are not only the use of a prohibited substance, but also its further destructive effect in the body.
Absorbed in the stomach almost instantly, it turns into formic acid and formaldehyde, which in small concentrations have a toxic effect on all organ systems, destroying cells and blocking their work. Since almost 90% of the substance is excreted by the kidneys, the urinary system is immediately affected. This is why consuming even small amounts of methyl alcohol is dangerous.
The functioning of the nervous system is disrupted, problems with the gastrointestinal tract appear, and with a large amount of the substance ingested, death quickly occurs.
Main symptoms of poisoning
Methyl alcohol is an extremely dangerous poison. Delay in providing first aid can be fatal, so everyone should know the main signs of intoxication with this substance. When they appear, you need to react quickly and try to cleanse the body of toxic compounds.
Doctors name several main symptoms that require special attention:
Nausea and vomiting. These signs signal that the body is trying to get rid of dangerous chemicals on its own. Along with the remnants of semi-digested food, part of the methyl alcohol will be removed from the stomach. This means that it will not be able to be fully absorbed into the bloodstream.- Abdominal pain. Discomfort or heaviness is another sign of acute intoxication. Sometimes, when poisoned, the sensations can become sharp and cutting.
- Headache. Migraines and attacks of dizziness are a consequence of central nervous system depression. The brain cannot function fully, since some of its neurons simply die under the influence of a toxic compound.
- Darkening before the eyes. Perhaps the main symptom of wood alcohol poisoning is blurred vision. First, the picture before the person’s eyes slowly blurs, then flies begin to flash. A little later, vision is completely lost. It is noteworthy that this function can rarely be restored. Even when saving the patient's life, they often remain blind.
- Blood pressure surges. Initially, the indicators increase and then drop sharply. All this indicates problems with the functioning of the heart. This muscular organ is damaged and can no longer pump the usual volume of blood.
Important! Events during methanol intoxication develop rapidly. As practice shows, in an hour it will be too late to do anything. It is for this reason that when drinking any alcohol you need to closely monitor your own well-being and the condition of other participants in the feast. Unfortunately, no one is safe from buying a fake.
Doctors also name a number of additional symptoms indicating possible poisoning. Thus, a person who drinks wood alcohol by mistake quickly becomes weaker. His speech is confused and his hearing is deteriorating. Then his body becomes covered in cold sweat, profuse salivation, tachycardia, and shortness of breath are observed. His behavior also changes.
Initially, the poison provokes attacks of aggression, which are later replaced by complete apathy and indifference to what is happening.
Ingestion
The destructive effect of methanol on the body often manifests itself when consuming denatured alcohol and other chemicals. Even ten milliliters of this “drink” pose a danger to humans, and a dose of thirty ml can be fatal. However, the same person can react differently to methanol if they use it constantly or at long intervals. Typically, people experience cyanosis (blue discoloration), deep and rare breathing, and possible convulsions. The pulse is rapid, the reaction of the pupils to light is absent. Most often, death occurs due to cessation of breathing. If a person is conscious, then he complains of stomach pain, spots flashing before his eyes, and vision problems. Even if the patient managed to survive in such a situation, the disturbances in liver function will be irreversible.
First aid to the victim
Doctors explain that poisoning with carbinol and its metabolic products is an extremely dangerous condition. If first aid is provided too late, the victim will lose consciousness, fall into a coma and die.
You can’t hesitate, but it’s impossible to carry out all the measures necessary for rehabilitation at home. For this reason, first of all, you need to call emergency doctors. By phone, you must clarify that there is a high probability of poisoning with industrial alcohol. Then doctors will already understand what they have to deal with.
Help for intoxication should be urgent. It is extremely important to stop the entry of toxins into the body. This means that you can no longer drink poisonous alcohol. Some of the alcohol that has already entered the stomach can be gotten rid of by provoking the gag reflex.
Doctors explain how to rinse the stomach if the risk of methanol intoxication is high. Plain water may not be effective, so dissolve a tablespoon of sodium bicarbonate in a liter of liquid. After this, the drink is taken orally warm, preferably in small sips. Next, use your fingers to press on the root of the tongue, provoking a gag reflex. This measure allows you to cleanse the stomach cavity of the poison present there.
Important! If a person begins to lose consciousness, then vomiting is induced with the help of other people. It is better to place the victim lying on his side and make sure that the remains of semi-digested food do not enter the respiratory tract.
If in case of ethanol poisoning it is appropriate to take sorbents - Smecta, Polysorb, Enterosgel, then in case of intoxication with a technical analogue such a measure is inappropriate. The substance leaves the digestive tract too quickly and enters the human blood. For this reason, intoxication develops almost instantly.
After freeing the stomach from a dangerous compound, you need to take care of cleansing the bloodstream. This can be done, but you need to choose the right antidote. Such a substance will be included in metabolism instead of wood alcohol and prevent the formation of highly toxic metabolites.
It is not difficult to find an antidote for methanol. It may be its closest analogue in the class of chemical compounds – ethanol. It is considered one of the main components of all alcoholic drinks, but weak alcohol is not suitable for relieving intoxication. It is not recommended to give the victim alcohol with additives.
The ideal option for neutralizing toxic effects is high-quality vodka. You need to drink 40 to 50 milliliters at a time. After this, the patient should be laid on his side, covered with a blanket and provided with fresh air. It is in this situation that you should expect an ambulance.
Usage
On an industrial scale, methanol is used as a solvent for varnishes and paints, as a raw material for subsequent chemical production, and as a substrate for medicines. It is part of antifreeze fluid. Methanol is most used in the production of formaldehyde and as an additive to gasoline, and it is also added to fuel for racing cars and motorcycles.
Many perfume companies use methanol in the production of perfumes for a more lasting aroma, but in the CIS this is prohibited by consumer rights law. In addition, methanol is also used in the production of gas and petroleum products. It acts on salts deposited on the walls of distillation pipes, thus maintaining their throughput.
Treatment of poisoning
Methanol is a dangerous poison, so if it is present inside the body, you should not refuse medical attention. The person must be hospitalized, and further treatment is carried out in a hospital setting.
Therapy, as a rule, comes down to neutralizing toxins. For this purpose, the victim is given a glass of vodka to drink every hour. Sometimes its quantity is increased to 100 milliliters. The required dose depends on the severity of symptoms and the general condition of the patient. There are times when the patient is unconscious. Treatment needs to be continued, so ethanol is administered to him intravenously using a dropper.
After the acute period ends, it is important to stabilize all vital functions of the body. You should not refuse such therapy, since only with its help can you at least partially restore your health. Further treatment will consist of several stages:
- Ethanol is introduced to neutralize methanol, but its breakdown products are much more difficult to deal with. An effective measure is forced diuresis with parallel alkalization of blood plasma.
- If muscle tissue is in a state of increased tone, and the patient is bothered by limb cramps, then specialized relaxing drugs are required - Sodium hydroxybutyrate or Sibazon.
- Wood alcohol can have a negative impact on metabolic processes within the body. To normalize them, vitamin therapy is carried out, and folic acid is considered its most important component.
Dangerous compounds come out through the kidneys. This organ takes the brunt of the load and is often damaged. In acute renal failure, hemodialysis is indicated.- The patient may have difficulty breathing. If the system does not function normally, then connection to a ventilator is required.
Of course, there is no need to talk about a quick recovery. Even if a person managed to save his life after drinking low-quality alcohol, it will take a lot of time to normalize the functioning of the entire body.
For full rehabilitation, you will need medications of different effects - vitamins; drugs that normalize blood pressure; hormonal substances.
Disposal
Methanol production products heavily pollute the environment. The water that is used in its purification and distillation is so saturated with harmful substances that even after repeated filtration, sedimentation and chemical purification, it still remains undrinkable. Therefore, at the moment, technologies for the production of methanol with a closed loop are being developed. This means that the water will be reused and not disposed of like biological or chemical waste. Considering the problem of fresh water on Earth, this method would help save valuable natural resources.
Consequences of intoxication
When consuming small doses of technical alcohol, mild poisoning is usually diagnosed. In this case, improvement in well-being occurs after 2-3 days. Rehabilitation may take longer if you drink too much dangerous liquid. Even if death can be avoided, there is a high probability that the patient's life will never be the same. As a rule, various negative consequences for the body are possible.
In first place in the number of diagnosed cases is complete or partial loss of vision. Darkening in the eyes and the appearance of a veil in front of them is one of the early symptoms of intoxication that you should pay attention to. Unfortunately, vision loss is often irreversible. This is due to damage not only to the eyes, but also to the nerves, as well as to the area of the cerebral cortex responsible for interpreting information received from the retina.
The second dangerous pathology is renal failure. This may occur due to the maximum load on the organ. Typically, the elimination of ethanol breakdown products is a function of the liver. The gland releases the necessary enzymes, and the toxins actually become harmless. Human kidneys are not used to playing such a role. Their cells are destroyed, which causes serious malfunctions. If acute deficiency is ignored, it will turn into a chronic form, which is even more difficult to treat.
Taking critical doses of poison almost always provokes death. It is not always possible to save a person. Often, all participants in a feast drink a dangerous liquid; accordingly, their health deteriorates simultaneously, and there is simply no one to help. The state of alcohol intoxication blocks the instinct of self-preservation, so many people simply ignore the alarming signals coming from the body.
Inhalation of vapors
Toxic fumes from formaldehyde rarely contain pure methanol. The effect on the human body when inhaled occurs in two stages. First, a reaction of local irritation of the mucous membranes occurs, and after absorption into the bloodstream, systemic poisoning of the body occurs. Clinical symptoms are as follows. The victim complains of dizziness, nausea, and a feeling of fog before the eyes due to severe intoxication. In addition, visual acuity sharply decreases, and pain appears in the right hypochondrium. Such poisoning is more often chronic than acute, since due to severe irritation of the upper respiratory tract, a person avoids contact with methanol.
Preventive measures
Methanol is a technical liquid, the use of which is prohibited in the food industry. Sometimes it ends up in low-quality alcohol, but, as a rule, large manufacturers try to strictly control all production processes. Purchasing drinks from trusted stores reduces the likelihood of poisoning to a minimum.
Methanol is rarely found in weak drinks. It contains ethanol, obtained naturally from the fermentation of plant materials. The situation is completely different with vodka. Sometimes unscrupulous manufacturers add cheap carbinol to reduce production costs. It's not easy to protect yourself. You should at least avoid purchasing alcohol from dubious, unlicensed stores.
Nowadays it is becoming increasingly difficult for manufacturers to counterfeit alcohol by reducing the share of ethanol. Industrial carbinol is usually tinted to make it easy to identify. This means that a bottle of a questionable drink will be different from ordinary clear vodka.
Any impurities should alert you and cause you to refuse to purchase or taste. In addition to foreign coloration, a sharp unpleasant odor may also appear, which will interrupt the ethanol vapors. This is another warning sign that should not be ignored.
If even a minimal amount of wood alcohol accidentally enters the body, emergency assistance is required. It is important to clear the stomach of excess poison as quickly as possible, give the victim an antagonist substance, and also call an ambulance.
Production
On an industrial scale, methanol can be produced in several ways. For the first time, the substance began to be obtained after dry distillation of wood in combination with lignin, then the technology for producing alcohol by decomposition of formic acid salts under the influence of high temperatures appeared. When this became unprofitable, it was decided to synthesize methanol from methane by incomplete oxidation. Now carbon dioxide and hydrogen are used for this with the addition of a copper-zinc catalyst.
Lethal dose
As has already been proven, the harm of large amounts of ethyl alcohol lies in poisoning the body. And like any poison, taken in a certain amount, it can cause death. The dangerous threshold depends on the characteristics (age, gender, body weight) of the person:
- For a healthy and non-drinking man, it can be 750 ml of vodka drunk over 5 hours or 300 ml of alcohol consumed over the same time.
- For a chronic alcoholic, a lethal dose is three bottles of vodka or 600 ml of alcohol, ingested in 5 hours or less.
Death can also occur with less alcohol if chronic diseases are present, especially in old age. The quality and quantity of food, the ability to self-purify, the rate of ethanol utilization and other factors play a role.
There cannot be methyl in alcoholic drinks
The ethyl alcohol molecule is very small, so it easily penetrates everywhere: it is not difficult to be absorbed by it. Having permeated the cellular structures of the brain, it causes its liquefaction, and once in the stomach, it penetrates many systems and tissues of the body. Intoxication begins.
But all the body’s defenses come to the fight, complex biochemical processes are launched, as a result of which more than 90 percent of ethyl alcohol is oxidized under the influence of a certain oxidizing agent, called NAD for short. However, it is required not to combat the effects of alcohol, but to help in the production of testosterone. And if a man is too keen on drinking alcohol, it means he doesn’t have enough of this important hormone in his blood.
It leads to:
- impotence;
- destruction of the glandular part of the prostate gland with subsequent disruption of its function;
- feminization, that is, the effeminacy of the male body.
If a nursing mother takes 100 ml of vodka at once, the ethanol will freely enter the mammary glands, and during the next feeding the baby may begin to experience a state of severe alcoholic intoxication.
In a pregnant woman, after drinking alcohol, ethanol enters the blood through diffusion into the circulatory system of the developing embryo, and malformations begin to develop, which in the medical world are called alcoholic embryopathy syndrome. In this case, the answer to the question of which alcohol is dangerous - ethyl or methyl alcohol - is obvious.
Cheap alcohol that brings death
Everyone understands perfectly well that good, high-quality alcohol cannot have a suspiciously low cost. And reasonable people allow themselves to buy a bottle of good alcohol only on holidays. What do alcohol dependent individuals and socially disadvantaged groups of the population do? They willingly consume any alcohol, and the cheaper it is, the better. And, of course, for every buyer there is a seller.
This is how a market for underground, surrogate (or burnt) alcohol appeared, which is sold at an extremely low price. Cheapness is formed due to the use of cheap alcohols, including methyl alcohols, in its production . But don’t think that only suspiciously cheap alcohol is potentially dangerous.
Death from intoxication occurs due to cardiac and respiratory arrest
Unfortunately, even among elite alcohol there are surrogates. Neither an attractive label nor the presence of a tax stamp is a guarantee of good quality. But still, expensive alcohol is counterfeited much less often; there are too many nuances that honest producers use to protect themselves from counterfeits.
More about the poison
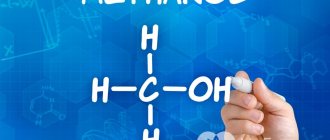
Methyl alcohol (or wood alcohol), also known as methanol (CH3OH) is an easily soluble, colorless liquid. The compound has a burning, very pungent taste and an aroma that hits the nose. Methanol vapors are extremely explosive and ignite instantly. When heated (increasing the temperature to +64-65⁰C), wood alcohol gives a violent reaction.
What is methanol
CH3OH is a substance necessary for humans. This substance has been successfully used for a long time in many industries . In particular, it is used in the following industries:
- chemical;
- perfumery;
- pharmaceutical.
CH3OH is used as a basis in the manufacture of various solvents, paints, and household liquids. It is also needed for medicine - blood tests are carried out using methanol.
Methyl alcohol is part of the group of poisonous narcotic compounds. Of all types of poisoning, poisoning with methyl alcohol is considered the most dangerous in terms of negative consequences.
Nomenclature
Let's consider three ways to form the name of a substance with the formula CH3OH. Historically, it is formed from the name of the hydrocarbon radical to which a hydroxyl group is attached. The CH3 radical is methyl, so the alcohol CH3OH is called methyl. According to the Geneva nomenclature, the suffix -ol is added to the name of the corresponding hydrocarbon - alkane. The compound will be called methanol. This name is the most common and is used quite often. In rational nomenclature, the compound we are considering is called carbinol.
see also
- Economics of methanol
- Iron complexes are found in the enzyme methane monooxygenase, which oxidizes methane to methanol, and in the important enzyme ribonucleotide reductase, which is involved in DNA synthesis.
- Messerschmitt Me.163 Comet is a German missile interceptor fighter of the Second World War. The Me-163 had a liquid-propellant rocket engine powered by 80 percent hydrogen peroxide and a liquid catalyst (potassium permanganate solution or a mixture of methanol, hydrazine hydrate, and water). In the combustion chamber, hydrogen peroxide decomposed to form a large volume of superheated vapor-gas mixture, creating powerful jet thrust.
Notes
- https://www.cdc.gov/niosh/npg/npgd0397.html
- M.M.Karavaev, V.E.Leonov, I.G.
Popov, E.T. Shepelev. Synthetic methanol technology. - Moscow: Chemistry, 1984. - 239 p. - Yurieva TM et al.
Mechanisms for hydrogenation of acetone to isopropanol and of carbon oxides to methanol over copper-containing oxide catalysts // Journal of Molecular Catalysis A: Chemical. - 1996. - Vol. 113, no. 3. - pp. 455-468. - Resolution of the Chief State Doctor of the Russian Federation dated July 11, 2007 N 47
- Biodiesel. Russian National Biofuel Association. Retrieved September 12, 2010.
- ↑ 1 2 3 4 Karakhanov E. A.
'ˆ '…‡Synthesis gas as an alternative to oil. II. Methanol and syntheses based on it // Soros educational journal. - 1997. - No. 12. - P. 68. (inaccessible link) - Waganer K. Mariculture on land
. - Biomass, 1981 - Ethanol and Energy Independence - Journey to Energy Independence
- Pierre Duret. New Generation of Engine Combustion Processes for the Future?
, 2002 - Internal Combustion Engines, Edward F. Obert, 1973
- Energy Citations Database (ECD) — — Document #6329346
- METHANOL (CAS Reg. No. 67-56-1), INTERIM ACUTE EXPOSURE GUIDELINE LEVELS (AEGLs) // EPA, 2005: “Odor: Alcoholic odor; pungent odor when crude; pungent "
- P. Carrer (1960), "Course in Organic Chemistry", p. 117.
- ↑ 1 2
Vale A (2007).
"Methanol". Medicine 35
(12):633–4. DOI:10.1016/j.mpmed.2007.09.014. - Methanol Poisoning Overview. Antizol. Archived from the original on October 5, 2011.
- https://www.epa.gov/chemfact/s_methan.txt "Humans - Ingestion of 80 to 150 mL of methanol is usually fatal to humans (HSDB 1994)."
- https://www.epa.gov/chemfact/s_methan.txt B. Acute Toxicity 2. Animals - Oral LD50
- Methanol (CASRN 67-56-1)
- GN 2.2.5.1313-03 - MPC of harmful substances in the air of the working area - www.dioxin.ru. www.dioxin.ru. Retrieved November 12, 2020.
- GOST 9805-84. Isopropyl alcohol. Technical conditions. This standard applies to isopropyl alcohol obtained by hydration of propylene.
- Nordoc.ru - GN 2.1.6.695-98. Maximum permissible concentrations (MPC) of pollutants in the atmospheric air of populated areas
- how to distinguish ethanol from methanol? — Chemists forum on XuMuK.ru
- ↑ 12
Acute poisoning - Electronic reference guide for emergency physicians. Chapter 15 - ALCOHOLD HYDROGENASE OF MAMMALS - AN OBJECT OF MOLECULAR MEDICINE Archived October 18, 2011. / Advances in biological chemistry, v. 43, 2003, p. 3-18
- Ferri Fred F.
Ferri's Clinical Advisor 2020: 5 Books in 1. - Elsevier Health Sciences, 2020. - P. 794. - ISBN 9780323448383. - How is “anti-freeze” dangerous for drivers’ health?
- ↑ 12
In the Czech Republic, a story of mass poisoning with counterfeit alcohol is being investigated - Os esquecidos do metílico (Galis.). Galicia Hoxe.
- Vuelve el caso del alcohol adulterado (Spanish). La Voz de Vigo. Retrieved December 20, 2020.
- Deaths From Illegal Liquor Rise to 308 in Southern India. The New York Times
(July 10, 1981). Retrieved December 20, 2020. - Hanumantharaya CH
. The Big Hooch Tragedy, Talk Magazine (14 December 2012). Archived from the original on January 4, 2020. Retrieved December 20, 2020. - Roberto Suro
. Italy acting to end the sale of methanol-tainted wine (April 9, 1986). Retrieved December 20, 2020. - Rupert Millar.
Italian methanol scandal.
thedrinksbusiness.com
(August 17, 2011). Retrieved December 20, 2020. - 122 salvadoreños mueren tras ingerir aguardiente adulterado con metanol (Spanish). El País (13 October 2000). Retrieved December 20, 2016.
- 10 días sin alcohol — Ley seca en El Salvador (Spanish). La Nación (13 October 2000). Retrieved December 20, 2020.
- Licor "Trueno" no contenía metanol, revela Fiscalía (Spanish). El Diario de Hoy (21 August 2001). Retrieved December 20, 2020.
- The number of deaths from Hawthorn poisoning in Irkutsk has risen to 78. Retrieved April 16, 2020.