Thallium is a silvery-white metal with a blue tint. It is impossible to recognize it by taste and smell. But it is unlikely that anyone will risk trying this substance. After all, thallium is a poison, with a high level of toxicity akin to lead and arsenic . Poisoning in large doses often ends in death. With gradual exposure to the body, it tends to accumulate in the kidneys, lungs, liver and brain. Leads to irreversible changes in the functioning of organs and systems and is extremely difficult to eliminate.
Thallium poisoning: signs and consequences
in the earth's crust (clark) 4.5? 10 -5% by mass, but due to extreme dispersion its role in natural processes is small. In nature, compounds of monovalent and, less frequently, trivalent T are found. Like alkali metals, T is concentrated in the upper part of the earth’s crust—in the granite layer (average content 1.5 ?
10–4%), in basic rocks it is less (2 × 10–5%), and in ultramafic rocks only 1 ? 10–6%. Only seven T. minerals are known (for example, cruxite, lorandite, vrbaite, etc.), all of them are extremely rare. T. has the greatest geochemical similarity with K, rb, cs, as well as with pb, ag, cu, bi. T. easily migrates in the biosphere. From natural waters it is sorbed by coals, clays, manganese hydroxides, and accumulates during water evaporation (for example, in Lake Sivash up to 5?
Physical and chemical properties. T. is a soft metal, easily oxidizes in air and quickly tarnishes. T. at a pressure of 0.1 MN/m2 (1 kgf/cm2) and a temperature below 233 °C has a hexagonal close-packed lattice (a = 3.4496 å; c = 5.5137 å), above 233 °C - a body-centered lattice cubic (a = 4.841 å), at high pressures 3.9 H/m 2 (39,000 kgf/cm 2) - face-centered cubic; density 11.85 g/cm3; atomic radius 1.71 å, ionic radii: tl + 1.49 å, tl 3+ 1.05 å; t pl 303.6 °C; boiling point 1457 °C, specific heat capacity 0.130 kJl (kg?
k ) [0.031 cal/g ? C) > at 20-100 °C; temperature coefficient of linear expansion 28? 10 –6 at 20 °C and 41.5? 10 –6 at 240–280 °C; thermal conductivity 38.94 W/(m?
K) [0.093 cal/(cm ? sec ? °C)]. Electrical resistivity at 0°C (18 × 10–6 ohm × cm); temperature coefficient of electrical resistance 5.177? 10 –3 – 3.98 ?
10 –3 (0–100 °C). The transition temperature to the superconducting state is 2.39 K. The temperature is diamagnetic, its specific magnetic susceptibility is 0.249? 10 –6 (30 °C).
Configuration of the outer electron shell of the atom tl 6 s 2 6 p 1 ; in compounds it has an oxidation state of +1 and +3. T. interacts with oxygen and halogens already at room temperature, and with sulfur and phosphorus when heated. It dissolves well in nitric acids, less so in sulfuric acids, and does not dissolve in hydrogen halides, formic, oxalic and acetic acids.
Does not interact with alkali solutions; freshly distilled water, which does not contain oxygen, has no effect on T. The main compounds with oxygen are oxide tl 2 o and oxide tl 2 o 3.
T. oxide and salts tl (i) nitrate, sulfate, carbonate - soluble; chromate, dichromate, halides (with the exception of fluoride), as well as T. oxide are slightly soluble in water. tl (iii) forms a large number of complex compounds with inorganic and organic ligands.
Tl(iii) halides are highly soluble in water. The connections tl (i) are of greatest practical importance.
Receipt. On an industrial scale, technical T.
obtained as a by-product from the processing of sulfide ores of non-ferrous metals and iron. It is extracted from semi-products of lead, zinc and copper production. The choice of raw material processing method depends on its composition.
For example, to extract metal and other valuable components from lead production dust, the material is sulfated in a fluidized bed at 300–350 °C. The resulting sulfate mass is leached with water and extracted from the solution with a 50% solution of tributyl phosphate in kerosene containing iodine, and then re-extracted with sulfuric acid (300 g/l) with the addition of 3% hydrogen peroxide.
The metal is isolated from re-extracts by cementation on zinc sheets. After melting under a layer of caustic soda, T. is obtained with a purity of 99.99%.
For deeper metal purification, electrolytic refining and crystallization purification are used.
Application. In technology, T. is used mainly in the form of compounds. Single crystals of solid solutions of halides tibr - tli and tlcl - tlbr (known in technology as KRS-5 and KRS-6) are used for the manufacture of optical parts in infrared devices; tlcl and tlcl-tlbr crystals - as radiators for Cherenkov counters.
tl 2 o is a component of some optical glasses; sulfides, oxysulfides, selenides, tellurides are components of semiconductor materials used in the manufacture of photoresistors, semiconductor rectifiers, and vidicons. An aqueous solution of a mixture of formic and malonic acid (heavy Clerici liquid) is widely used to separate minerals by density. T. amalgam, which hardens at –59 °C, is used in low-temperature thermometers. Metal T. is used to produce bearing and low-melting alloys, as well as in oxygen meters to determine oxygen in water.
204tl is used as a source of b-radiation in radioisotope devices.
Thallium in the body. T. is constantly present in the tissues of plants and animals. In soils its average content is 10–5%, in sea water 10–9%, in animal organisms 4? 10–5%. In mammals, T. is well absorbed from the gastrointestinal tract, accumulating mainly in the spleen and muscles.
In humans, the daily intake of T. from food and water is about 1.6 mcg, and from air - 0.05 mcg. The biological role of T. in the body has not been clarified. Moderately toxic to plants and highly toxic to mammals and humans.
Poisoning by T. and its compounds is possible during their production and practical use. T. enters the body through the respiratory organs, intact skin and digestive tract.
It is excreted from the body over a long period of time, mainly through urine and feces. Acute, subacute and chronic poisoning have a similar clinical picture, differing in the severity and speed of onset of symptoms. In acute cases, after 1-2 days, signs of damage to the gastrointestinal tract (nausea, vomiting, abdominal pain, diarrhea, constipation) and respiratory tract appear. After 2-3 weeks, hair loss and symptoms of vitamin deficiency (smoothing of the mucous membrane of the tongue, cracks in the corners of the mouth, etc.) are observed.
d.). In severe cases, polyneuritis, mental disorders, visual impairment, etc. may develop. Prevention of occupational poisoning: mechanization of production processes, sealing of equipment, ventilation, use of personal protective equipment.
Lit.: Chemistry and technology of rare and trace elements, ed. K. A. Bolshakova, vol. 1, [M., 1965]; 3elikman A. N., Meerson G. A., Metallurgy of rare metals, M., 1973; Thallium and its application in modern technology, M., 1968; Tikhova G.
S., Darvoyd T.I., Recommendations for industrial sanitation and safety precautions when working with thallium and its compounds, in the collection: Rare Metals, v. 2, M., 1964; Bowen N.y. M., trace elements in biochemistry, l.-ny, 1966.
Israelson Z.I., Mogilevskaya O.Ya., Suvorove. V. Issues of occupational hygiene and occupational pathology when working with rare metals, M., 1973.
Prevention
Thallium poisoning is most often observed in employees of industries in which this metal or its compounds are used in the technological process. Therefore, to prevent them, it is necessary to establish careful monitoring of employee compliance with safety regulations.
When working in a dangerous area, it is necessary to use personal protective equipment (respirators, gloves, special shoes and overalls). After finishing their shift, employees must take off their work clothes and hand them over to the laundry, and be sure to take a shower.
To prevent thallium poisoning, you should not smoke, drink water or eat food in the workplace.
All employees who come into contact with thallium should undergo regular medical examinations. If signs of poisoning are detected, they are subject to immediate hospitalization and are prohibited from working in the hazardous area in the future.
Education: graduated from the Tashkent State Medical Institute with a degree in general medicine in 1991. Repeatedly took advanced training courses.
Work experience: anesthesiologist-resuscitator at a city maternity complex, resuscitator at the hemodialysis department.
The information is generalized and is provided for informational purposes. At the first signs of illness, consult a doctor. Self-medication is dangerous to health!
Dentists appeared relatively recently. Back in the 19th century, pulling out diseased teeth was the responsibility of an ordinary hairdresser.
More than $500 million a year is spent on allergy medications in the United States alone. Do you still believe that a way to finally defeat allergies will be found?
You are more likely to break your neck if you fall off a donkey than if you fall off a horse. Just don't try to refute this statement.
A person taking antidepressants will, in most cases, become depressed again. If a person has coped with depression on his own, he has every chance to forget about this condition forever.
Four pieces of dark chocolate contain about two hundred calories. So if you don’t want to gain weight, it’s better not to eat more than two slices a day.
When lovers kiss, each of them loses 6.4 calories per minute, but at the same time they exchange almost 300 types of different bacteria.
Ten deadly poisons and their effects on humans
by the characteristic green pine in the spectrum (tallos - green bud). The chemical properties of thallium are determined by its belonging to the secondary group of a-transition metals of group III elements of the periodic table.
The atomic weight of thallium is 204.39, atomic number 81, density 11.85 g/cm°. Melting point 303 C, boiling point 1460 ° C.
The vapor pressure of thallium at a temperature of 825°C is 1, at 983°C - 10, at 1040°C - 20. at 1457°C - 760 mmHg. Art. In chemical compounds, it acts as a monovalent or trivalent metal, forming two types of compounds - oxide and oxide. In air, thallium becomes covered with a film of nitrous oxide; at 100°C it quickly oxidizes to form TI2O and Tl2O3. Reacts with chlorine, bromine and iodine at room temperature. When interacting with alcohols, it forms alcoholates.
Easily dissolves in HNO3. There are salts of both mono- and trivalent thallium (V.K. Grigorovich, 1970). Thallium is a rare trace element. The nature of its distribution in nature is determined by the proximity in chemical properties and sizes of ionic radii to alkali metals, as well as to calcophile elements.
Commercial sulfide concentrates (sphalerite, galena, pyrite and marxcite) are of industrial importance as sources of raw materials for the production of thallium. Thallium is not extracted directly from ores and concentrates containing it in quantities not exceeding thousandths of a percent.
The raw materials for its industrial production are waste and intermediate products from the production of non-ferrous metals. The thallium content in these materials varies widely (from hundredths of a percent to whole percent) and depends not only on the thappium content in the feedstock, but also on the nature of production and the adopted technology for obtaining the base metal.
Thus, the extraction of thallium is associated with complex processing of raw materials and is carried out along the way with the production of other metals. When the concentration of thallium in the processed raw materials is low, the technology for its production at the first stage usually comes down to the production of tappium concentrate, which is then processed into industrial metal or its substances.
In the Soviet Union, thallium production was organized at a number of lead and zinc plants (T.I. Darvoyd et al., 1968).
Thallium oxides
There are 3 known compounds of thallium with oxygen: oxide - Tl2O, oxide - Tlg2O3 and peroxide -Tl2O3 (little studied).
One of the chemical elements that belongs to the metal group is thallium. Thallium is always present in the human body in small quantities. Despite this, contact with it should be avoided, as it leads to severe intoxication. Thallium has a poisonous effect on humans.
History [edit | edit code]
Thallium was discovered by the spectral method in 1861 by the English scientist William Crookes in the sludge of lead chambers of a sulfuric acid plant near the city of Abberode, located in the Harz mountain range.
Thallium metal was independently obtained by William Crookes and French chemist Claude-Auguste Lamy in 1862 [5].
Origin of the name [edit | edit code ]
The element received its name from the characteristic green lines of its spectrum and the green color of the flame. From ancient Greek. θαλλός - young, green branch [6].
What is thallium and possible methods of poisoning with it?
Before you find out where you can get poisoned, you need to answer the question: thallium salts - what are they? This is a potent toxin that affects the peripheral and central nervous system, kidneys, and gastrointestinal tract. In industry it is used much less frequently than other metals. It is important to remember that any contact with it in most cases ends in death, since it is a potent poison.
During numerous experiments, this chemical element was identified in the human body in greater quantities in fatty tissues. To this day, its functions and purpose in our body remain a big mystery. There is a minimal amount of it in plants. Therefore, scientists believe that thallium (or thallium) enters the human body through plant products. The concentration is so low that it does not cause any harm to health.
It is important to know where the poison is located. Intoxication can occur in one of the following situations:
- Working with pesticides or insecticides. Most poisonings occur in agricultural workers.
- While working in production where waist is used. For example, the production of pyrotechnics, thermometers, fluorescent paints and light bulbs.
- The metal is part of the poison for rodents, so poisoning can occur during the treatment of the premises against rodents.
- Most often, children are poisoned by ingesting a product that contains thallium pesticides. It is so important to hide all poisons, solutions and chemicals from the child, because he still does not know anything about the upcoming danger. For a small organism, even the smallest amount of such a substance can be the last.
When working with thallium, be sure to wear a respirator and a protective suit. For poisoning, skin contact is not always necessary; it is enough for thallium sulfate to enter the body through the respiratory tract. Very often this chemical element is used to intentionally kill a person.
Links
| in Wiktionary |
| in Wikiquote |
| in Wikisource |
- [www.nt.org/ri/gd/yd.htm Ida Danilovna Gadaskina “Poisons - yesterday and today: Essays on the history of poisons”]
- [www.vokrugsveta.ru/vs/?article_id=1373 Magazine “Around the World”: Poisonous evolution]
- [www.stopusa.be/scripts/texte.php?section=CL&langue3&id=24471 Spraying of Agent orange by US Army in Vietnam and its consequences] (English)
- [www.monde-solidaire.org/spip/article.php3?id_article=2295 Epandage de l'Agent Orange par l'US Army au Viêt Nam et ses conséquences] (French)
- [essuir.sumdu.edu.ua/handle/123456789/4836 Metabolic poisons and blood vessels]
| This is a draft article on toxicology. You can help the project by adding to it. |
The effect of thallium on the body
We found out above that this metal has 3 ways of entering the body:
- skin contact,
- through the digestive system,
- through the respiratory tract.
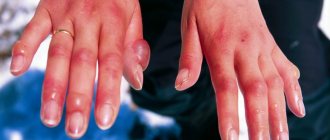
Thallium has a toxic effect on the human body. 1 gram is enough for poisoning. Larger amounts lead to death. The fastest and most severe poisoning occurs in situations where thallium is ingested. Getting it into the stomach leads to local inflammation. Less than an hour is enough for this poison to spread throughout the body. The kidneys suffer the most, since only they are able to remove it from the body. Namely, their functions are disrupted, since thallium settles on the internal walls of the organs. It is eliminated from the body very, very slowly. It will take up to 3 months to completely cleanse the body of a small amount of poison.
Not only the kidneys, but also all other vital organs suffer from intoxication. Metal deposition is observed in the heart, nerve cells of the brain, liver, nerve pathways and blood vessels. In more severe cases, swelling of the brain is observed. As a rule, this is what causes all deaths during poisoning.
Possible complications
Severe thallium poisoning is fatal. But even milder poisonings have an unfavorable prognosis, as they lead to numerous long-term consequences:
- tremor of the limbs;
- ataxia;
- memory impairment;
- stroke;
- myocardial infarction;
- hormonal disorders;
- chronic lung diseases;
- chronic diseases of the digestive system;
- infertility;
- disorders of potency in men and menstrual function in women;
- increased risk of congenital pathologies in offspring.
Manifestations of thallium poisoning
The complexity of intoxication directly depends not only on the amount of poison taken, but also on the age of the victim and his weight. A child needs much less time and amount of chemical to develop poisoning.
After the first 2 hours, you can observe how the first symptoms begin to appear. The general condition of the victim begins to quickly deteriorate and after this time the full clinical picture can be observed. The first symptoms of thallium poisoning:
- Acute abdominal pain that spreads quickly. At this moment, all parts of the intestines and stomach are affected.
- Nausea followed by vomiting. Typically, vomit consists of gastric juice, bile and food debris.
- Due to intestinal damage, diarrhea occurs, which is accompanied by blood. This is caused by bleeding in the intestines.
- A rapid heart rate or tachycardia can soon lead to a permanently disturbed heart rhythm.
- Frequent breathing.
- There is a drop in blood pressure. This is caused by internal bleeding in the intestinal area.
If you do not seek medical help in time, the following symptoms will appear over the next week:
- seizures that closely resemble epilepsy,
- severe and persistent headache in one part of the head,
- apathy, pronounced weakness of the whole body,
- myalgia, so-called muscle pain, which is localized in the lower extremities,
- staggering, poor coordination, especially noticeable when walking. This suggests that thallium damaged the cerebellum,
- inflammation of the nerve tracts or polyneuritis, which manifests itself as pain throughout the body,
- a sharp deterioration in vision, in particularly advanced and severe forms, complete blindness occurs, which indicates damage to the visual center in the brain,
- loss of consciousness, deep comatose states are observed.
In cases of acute and severe poisoning, the victim dies within the first 24 hours due to cerebral edema or internal intestinal bleeding.
Sarin gas
Sarin is one of the most dangerous and deadly nerve gases
, which is hundreds of times more toxic than cyanide. Sarin was originally produced as a pesticide, but the clear, odorless gas soon became a powerful chemical weapon.
A person can be poisoned by sarin gas by inhaling or exposing the gas to the eyes and skin. Initial symptoms may include a runny nose and chest tightness, difficulty breathing and nausea.
.
Then the person loses control over all functions of his body and falls into a coma, convulsions and spasms occur until suffocation occurs.
First aid for poisoning
If there is a suspicion that thallium intoxication has occurred, it is necessary to call an ambulance without waiting for the first symptoms. After all, every minute is important. First aid and further treatment are carried out only by medical staff in a hospital setting.
All you can do is provide the correct first aid. In this way, you will remove the collected particles of thalia in the stomach and reduce the severity of the entire further poisoning process. You can carry out the following activities yourself:
- Stomach cleansing. If the poison was swallowed, then this activity is recommended to be carried out in the first minutes. To do this, the victim needs to drink more than 1 liter of plain water in one gulp, and then induce a gag reflex. In order to provoke gagging, press on the root of the tongue. It is necessary to repeat this procedure several times. This will help remove the maximum amount of poison from the stomach. If there is a disturbance of consciousness, then such washing is strictly prohibited. It should also be stopped in cases where the vomit is dark or even black in color. This color can only indicate that internal bleeding has begun. And rinsing will only strengthen it and increase the volume of blood loss.
- Sorbents. It's worth looking into the first aid kit. Perhaps there will be drugs from the sorbent group. Read the instructions carefully to understand what dosage the patient needs to take. For example, 1 tablet of activated carbon is needed per 10 kg of weight.
- Drink. It should be plain water. Drinking will help relieve dehydration that may occur during poisoning. You should pay attention to the water temperature. It should be room temperature, never hot. Carbonated drinks should also be avoided.
Only doctors who arrive on call can provide first aid. It consists of the following activities:
- drugs are administered that eliminate breathing and heartbeat disorders,
- special droppers are placed that relieve intoxication syndrome,
- in case of severe intestinal bleeding, hemostatic drugs are administered,
- if uncontrollable vomiting is observed, then antiemetics are given,
- small children or victims with impaired consciousness undergo gastric lavage through a tube.
Once all vital signs have stabilized, he is taken to the nearest hospital. There, hospitalization is carried out in the toxicology department or in intensive care.
Excerpt describing Poison
- Here. What lightning! - they were talking. In the abandoned tavern, in front of which stood the doctor’s tent, there were already about five officers. Marya Genrikhovna, a plump, fair-haired German woman in a blouse and nightcap, was sitting in the front corner on a wide bench. Her husband, a doctor, was sleeping behind her. Rostov and Ilyin, greeted with cheerful exclamations and laughter, entered the room. - AND! “What fun you are having,” Rostov said, laughing. - Why are you yawning? - Good! That's how it flows from them! Don't wet our living room. “You can’t dirty Marya Genrikhovna’s dress,” answered the voices. Rostov and Ilyin hurried to find a corner where they could change their wet dress without disturbing Marya Genrikhovna’s modesty. They went behind the partition to change clothes; but in a small closet, filling it completely, with one candle on an empty box, three officers were sitting, playing cards, and did not want to give up their place for anything. Marya Genrikhovna gave up her skirt for a while to use it instead of a curtain, and behind this curtain Rostov and Ilyin, with the help of Lavrushka, who brought packs, took off the wet dress and put on a dry dress. A fire was lit in the broken stove. They took out a board and, having supported it on two saddles, covered it with a blanket, took out a samovar, a cellar and half a bottle of rum, and, asking Marya Genrikhovna to be the hostess, everyone crowded around her. Some offered her a clean handkerchief to wipe her lovely hands, some put a Hungarian coat under her feet so that it would not be damp, some curtained the window with a cloak so that it wouldn’t blow, some brushed the flies off her husband’s face so that he would not wake up. “Leave him alone,” said Marya Genrikhovna, smiling timidly and happily, “he’s already sleeping well after a sleepless night.” “You can’t, Marya Genrikhovna,” the officer answered, “you have to serve the doctor.” That’s it, maybe he’ll feel sorry for me when he starts cutting my leg or arm. There were only three glasses; the water was so dirty that it was impossible to decide whether the tea was strong or weak, and there was only enough water in the samovar for six glasses, but it was all the more pleasant, in turn and by seniority, to receive your glass from Marya Genrikhovna’s plump hands with short, not entirely clean, nails . All the officers seemed to really be in love with Marya Genrikhovna that evening. Even those officers who were playing cards behind the partition soon abandoned the game and moved on to the samovar, obeying the general mood of courting Marya Genrikhovna. Marya Genrikhovna, seeing herself surrounded by such brilliant and courteous youth, beamed with happiness, no matter how hard she tried to hide it and no matter how obviously shy she was at every sleepy movement of her husband, who was sleeping behind her. There was only one spoon, there was most of the sugar, but there was no time to stir it, and therefore it was decided that she would stir the sugar for everyone in turn. Rostov, having received his glass and poured rum into it, asked Marya Genrikhovna to stir it. - But you don’t have sugar? - she said, still smiling, as if everything that she said, and everything that others said, was very funny and had another meaning. - Yes, I don’t need sugar, I just want you to stir it with your pen. Marya Genrikhovna agreed and began to look for a spoon, which someone had already grabbed. “You finger, Marya Genrikhovna,” said Rostov, “it will be even more pleasant.” - It's hot! - said Marya Genrikhovna, blushing with pleasure. Ilyin took a bucket of water and, dripping some rum into it, came to Marya Genrikhovna, asking him to stir it with his finger. “This is my cup,” he said. - Just put your finger in, I’ll drink it all. When the samovar was all drunk, Rostov took the cards and offered to play kings with Marya Genrikhovna. They cast lots to decide who would be Marya Genrikhovna's party. The rules of the game, according to Rostov’s proposal, were that the one who would be king would have the right to kiss Marya Genrikhovna’s hand, and that the one who would remain a scoundrel would go and put a new samovar for the doctor when he woke up. - Well, what if Marya Genrikhovna becomes king? – Ilyin asked. - She’s already a queen! And her orders are law. The game had just begun when the doctor’s confused head suddenly rose from behind Marya Genrikhovna. He had not slept for a long time and listened to what was said, and, apparently, did not find anything cheerful, funny or amusing in everything that was said and done. His face was sad and despondent. He did not greet the officers, scratched himself and asked permission to leave, as his way was blocked. As soon as he came out, all the officers burst into loud laughter, and Marya Genrikhovna blushed to tears and thereby became even more attractive in the eyes of all the officers. Returning from the yard, the doctor told his wife (who had stopped smiling so happily and was looking at him, fearfully awaiting the verdict) that the rain had passed and that she had to go spend the night in the tent, otherwise everything would be stolen.
Examination and treatment of the victim
It is not easy to detect thallium in the body. To do this, the abdominal cavity is examined X-ray. It can be seen in the picture because it does not allow X-rays to pass through. It may collect in the kidney or intestinal area.
Thallium poisoning is very serious, so treatment begins within minutes of hospitalization. It consists of the following components:
- Dithiocarb is introduced - this is an antidote for thallium. Thanks to it, toxins are neutralized and removed from the body. But improvement doesn't happen overnight.
- Hemodialysis also helps remove toxins from the body. It is carried out on the first day of poisoning. Helps prevent acute kidney failure.
- If there is no intestinal bleeding, then laxatives are used.
- Medicines are administered to normalize and maintain blood pressure and heart function.
- Droppers, which are aimed at normalizing blood pressure and reducing intoxication. Any drug is administered under strict control of the electrolyte composition of the blood.
Being in nature [edit | edit code ]
Thallium is a trace element. Contained in blende and pyrites of zinc, copper and iron, in potassium salts and mica. Thallium is a heavy metal. Only seven thallium minerals are known (cruxite (Cu, Tl, Ag)2Se, lorandite TlAsS2, vrbaite Tl4Hg3Sb2As8S20, hutchinsonite (Pb, Tl)S • Ag2S • 5As2S5, avicennite Tl2O3), all of them are extremely rare. The main mass of thallium is associated with sulfides and primarily with iron disulfides. In pyrite, it was found in 25% of the analyzed samples. Its content in iron disulfides is often 0.1-0.2%, and sometimes reaches 0.5%. In galena, the thallium content ranges from 0.003 to 0.1% and rarely more. High concentrations of thallium in disulfides and galenas are characteristic of low-temperature lead-zinc deposits in limestones. Thallium content reaching 0.5% is observed in some sulfosalts. Small amounts of thallium are found in many other sulfides, for example, in sphalerites and chalcopyrites of some copper pyrite deposits, the content ranges from 25 to 50 g/t. Thallium has the greatest geochemical similarity with K, Rb, Cs, as well as with Pb, Ag, Cu, Bi. Thallium easily migrates in the biosphere. From natural waters it is sorbed by coals, clays, manganese hydroxides, and accumulates during water evaporation (for example, in Lake Sivash up to 5⋅10−8 g/l). Contained in potassium minerals (mica, feldspars), sulfide ores: galena, sphalerite, marcasite (up to 0.5%), cinnabar. It is present as an impurity in natural oxides of manganese and iron [7].
Average thallium content (by weight):
- in the earth's crust 4.5⋅10 −5%;
- in ultramafic rocks 10−6%;
- in basic rocks 2⋅10 −5%;
- in sea water 10−9%.
What could be the consequences?
In all cases, it is noted that intoxication with thallium sulfate never goes away without a trace, regardless of whether assistance was provided in a timely and correct manner or after some time. As a rule, the consequences last a lifetime. In more complex poisonings, after recovery there is complete loss of ability to work. The most common consequences after intoxication:
- Alopecia. This is typical for both men and women. Hair loss can be partial, or it can be complete baldness. As a rule, this consequence is irreversible.
- The retina of the eye atrophies. This leads to complete or partial loss of vision.
- In men, impotence, and in women, the menstrual cycle is disrupted, and infertility is possible.
- Kidney failure is caused by damage to the kidneys, in some cases the victim needs constant hemodialysis.
- Skin atrophy, dermatitis, rashes and redness.
- Heart failure, which becomes chronic.
- Depression.
- Memory impairment.
- Development of epilepsy.
Due to the fact that thallium is not such a common metal, poisoning with it is rare, but it is much more serious than others. It is important to remember what thallium salts are and where the poison is found. Most often, children who have consumed a substance that contains thallium sulfate, or people who work in production using it, suffer. To save life, it is necessary to call an ambulance at the slightest suspicion of intoxication. Doctors have to deal with a large number of complications that may arise, so the period of treatment and rehabilitation is very long. Even with timely first aid, the victim may remain disabled forever.
Tetrodotoxin
This deadly poison is found in the organs of puffer fish.
, from which the famous Japanese delicacy “fugu” is prepared. Tetrodotoxin persists in the skin, liver, intestines and other organs, even after the fish has been cooked.
This toxin causes paralysis, seizures, mental disorder
and other symptoms. Death occurs within 6 hours after ingestion of the poison.
Every year, several people are known to die painful deaths from tetrodotoxin poisoning after eating fugu.
Receiving [edit | edit code ]
Technically pure thallium is purified from other elements contained in flue dust (Ni, Zn, Cd, In, Ge, Pb, As, Se, Te) by dissolving in warm dilute acid, followed by precipitation of insoluble lead sulfate and the addition of HCl to precipitate thallium chloride (TlCl). Further purification is achieved by electrolysis of thallium sulfate in dilute sulfuric acid using platinum wire, followed by melting of the liberated thallium in a hydrogen atmosphere at 350–400 °C.
Properties [edit | edit code ]
Thallium is a shiny silvery soft metal with a bluish tint. In air it quickly fades, becoming covered with a black film of thallium oxide Tl2O [3]. In water in the presence of oxygen it dissolves to form TlOH; in the absence of oxygen it does not react, so thallium is stored under a layer of boiled distilled water (or paraffin, and also varnished) [3].
Physical properties [edit | edit code ]
Exists in three modifications. The low-temperature modification Tl II is a crystal of the hexagonal system, space group P
63/
mmc
, cell parameters
a
= 0.34566 nm,
c
= 0.55248 nm,
Z
= 2, magnesium type lattice.
Above 234 °C there is a high-temperature modification Tl I of the cubic system (body-centered lattice), space group Im
3
m
, cell parameters
a
= 0.3882 nm,
Z
= 2, α-Fe type lattice;
the enthalpy of transition between modifications I and II is 0.36 kJ/mol. At 3.67 GPa and 25 °C - Tl III modification of the cubic system (face-centered lattice), space group Fm
3
m
, cell parameters
a
= 0.4778 nm,
Z
= 4. The melting point is 577 K (304 °C), boils at 1746 K (1473 °C) [2]. Thallium belongs to the group of heavy metals; its density is 11.855 g/cm 3 [2].
The cross section for the capture of thermal neutrons by an atom is 3.4 ± 0.5 barn. The configuration of the outer electrons is 6s 2 6p. Ionization energies (in eV): Tl 0 →Tl + →Tl 2+ →Tl 3+ →Tl 4+ are respectively equal to 6.1080; 20.4284; 29.8; 50.0 [3] .
Thallium is diamagnetic, the mass magnetic susceptibility of polycrystalline hexagonal thallium is χ = −0.249 10 −9 m 3 /kg under normal conditions, −0.258 10 −9 m 3 /kg at T
= 14.2 K.
For cubic polycrystalline thallium at T
> 235 K, the mass magnetic susceptibility is −0.158·10−9 m 3 /kg. Single-crystal hexagonal thallium exhibits anisotropy, χ|| = −0.420·10 −9 m 3 /kg, χ⊥ = −0.164·10 −9 m 3 /kg. For liquid thallium χ = −0.131·10 −9 m 3 /kg at the melting temperature [8].
At a temperature of 2.39 K, thallium enters the superconducting state.
The spectrum of thallium in the visible range has a bright line with a wavelength of 525.046 nm (green), from which this element got its name.
Chemical properties [edit | edit code ]
Reacts with non-metals: with halogens and oxygen at room temperature, with sulfur, selenium, tellurium, phosphorus - when heated. It fuses with arsenic without forming a compound. It does not react with hydrogen, nitrogen, carbon, silicon, boron, as well as with ammonia and dry carbon dioxide.
Application [ edit | edit code ]
- Thallium amalgam has a low melting point ( t
pl = −61 °C; only the eutectic in the sodium-potassium-cesium system with
t
pl = −78 °C is more fusible). It is used for filling low-temperature thermometers and as a coolant. - Nuclide 201 Tl is used in medicine for cardiac research.
- Thallium is introduced as an activator into sodium iodide crystals, which are used as a scintillator for recording ionizing radiation.
- In infrared optics, thallium(I) bromide and iodide are used as lens materials. In addition, during the Great Patriotic War, thallium oxysulfide (thallophide) was used as a sensitive element in night vision devices [9].
- Thallium(I) iodide is added to metal halide lighting lamps.
- Clerici's solution, consisting of thallium formate (HCOOTl) and thallium malonate (CH2(COOTl)2), is used in mineralogy to determine the properties of minerals.
- Thallium(I) sulfate and thallium(I) carbonate have previously been used as a rodent control agent in hard-to-reach areas.
- Thallium is the main component of some relatively strong
oxidizing reagents in organic synthesis:
- Thallium(III) trifluoroacetate or thallium tris(trifluoroacetate), TTFA (Tl(Otfac)3); [10]
- Thallium trinitrate, TTN (Tl(NO3)3);
- Thallium triacetate, TTA (Tl(CH3COO)3).
The health of your planet is in your hands!
| Appearance of a simple substance | |
| Soft silvery-white metal with a bluish tint | |
| Properties of the atom | |
| Name, symbol, number | Thallium (Tl), 81 |
| Atomic mass (molar mass) | [204,382; 204.385] [comm 1] [1] a. e.m. (g/mol) |
| Electronic configuration | [Xe] 4f 14 5d 10 6s 2 6p 1 |
| Atomic radius | 171 pm |
| Chemical properties | |
| Covalent radius | 148 pm |
| Ion radius | (+3e) 95 (+1e) 147 pm |
| Electronegativity | 1.62 (Pauling scale) |
| Electrode potential | Tl←Tl + −0.338 V Tl←Tl 3+ 0.71 V |
| Oxidation states | 3, 1 |
| Ionization energy (first electron) | 588.9 (6.10) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 11.849 [2] g/cm³ |
| Melting temperature | 577 K (304 °C, 579 °F) [2] |
| Boiling temperature | 1746 K (1473 °C, 2683 °F) [2] |
| Ud. heat of fusion | 4.31 kJ/mol |
| Ud. heat of vaporization | 162.4 kJ/mol |
| Molar heat capacity | 26.3 [3] J/(K mol) |
| Molar volume | 17.2 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | hexagonal |
| Lattice parameters | a=3.456 c=5.525 [4] |
| c / a ratio | 1,599 |
| Debye temperature | 96.00 K |
| Other characteristics | |
| Thermal conductivity | (300 K) 38.9 [3] W/(m K) |
| CAS number | 7440-28-0 |
| 4f 14 5d 10 6s 2 6p 1 | |
| Crystal structure: hexagonal Thallium (lat. thallium), tl, chemical element of group III of the periodic system of Mendeleev, atomic number 81, atomic mass 204.37; on a fresh cut there is gray shiny metal; refers to rare trace elements. In nature, the element is represented by two stable isotopes 203 tl (29.5%) and 205 tl (70.5%) and radioactive isotopes 207 tl - 210 tl - members of the radioactive series. The radioactive isotopes 202 tl (t 1/2 = 12.5 days), 204 tl (t 1/2 = 4.26 years) and 206 tl (t 1/2 = 4.19 min) were artificially obtained. T. was discovered in 1861 by W. Crookes in the sludge of sulfuric acid production using the spectroscopic method based on the characteristic green line in the spectrum (hence the name: from the Greek thall os - young, green branch). In 1862, the French chemist C. O. Lamy first isolated T. and established its metallic nature. Distribution in nature. Average T content. |