Good afternoon, many will be interested in understanding their health and their loved ones, and I will tell you my experience, and we will talk about First aid for poisoning with acids and alkalis. Most likely, some details may differ, as was the case with you. Please note that you should always consult with highly specialized specialists and not self-medicate. Naturally, you can quickly find the answer to the simplest questions and diagnose yourself. Write your questions/suggestions in the comments, and together we will improve and supplement the quality of the material provided.
Poisoning with acids and alkalis most often occurs when they are used in everyday life. In most cases, intoxication occurs when using acetic acid, less often - alkalis and oxidizing agents. The mentioned substances cause a chemical burn: when they come into contact with the skin, the epidermis is completely destroyed. Penetration of toxic agents into the stomach can cause cardiac arrest.
Causes of poisoning with acid compounds
The greatest danger of acid poisoning awaits people who come into contact with them in everyday life.
Alas, the overwhelming majority of all acute intoxications caused by acids getting on the skin or inside the body occur in cases of careless and careless handling of aggressive substances.
The reasons are:
- repair work;
- processing of plants and horticultural crops;
- cleaning clothes, shoes, home textiles and furniture;
- accidental ingestion;
- non-compliance with food preparation standards (acetic acid);
- inhalation of vapors;
- industrial accidents;
- attempted murder or suicide.
People who are intoxicated may accidentally swallow the essence instead of vodka.
Most often, poisoning occurs in an acute form, when an aggressive substance enters the body once and in a large volume. But chronic intoxication is not uncommon; most often it is observed in people whose professional activities are in one way or another connected with the chemical industry, the production of metals, dyes, explosive components, and fertilizers.
IMPORTANT! Any composition that contains acid, be it household chemicals or garden fertilizers, is potentially dangerous not only for people, but also for animals.
Application area
The alkali dissolves in water or alcohol and produces quantitative heat. As a result of the reaction, salts are formed, which are used in industry.
The scope of use is heterogeneous, often used in the following industries:
- Life and cosmetology;
- Fisheries: disinfection and cleaning of ponds;
- Agricultural economy: production of fertilizers;
- Medicine;
- Use instead of electrolytes.
Alkali and acid are substances that can cause death if poisoned.
Substances containing alkali are used carefully and in compliance with safety measures. Alkali poisoning is extremely dangerous for humans and causes serious harm to health. The cellular protein is affected and burns are formed. The result is a disruption of the functioning of well-coordinated body systems.
Sulfuric acid: symptoms, first aid
The chemical formula of sulfuric acid is familiar to almost everyone from a school chemistry course.
This odorless oily substance has no color when purified, and when unpurified it takes on a yellowish light tint. It is used in the manufacture of dyes, chemical fibers, and is found in the leather industry, the oil industry, and sometimes in the food industry. The main area of application is the production of various fertilizers.
ATTENTION! The norm for the content of sulfuric acid in the air is 1 mg/m2, in the open air - 3 mg/m2, in water - 0.008 mg/l. The slightest excess of concentration is fraught with poisoning.
Signs of intoxication
Unlike damage from gases, food poisons or drugs, which sometimes cause vague symptoms, sulfuric acid poisoning is accompanied by extremely pronounced symptoms.
| Entry into the esophagus | Inhalation of vapors | Skin contact |
| Sharp, sharp pain in the stomach and esophagus | Respiratory tract burns | Sharp pain in the affected area |
| Severe drooling | Labored breathing | Appearance of a loose, light-colored crust on the skin |
| Intense vomiting with bile and blood, accompanied by severe pain | Redness of the eyes | Gradual change in skin color from white to gray, then to brown |
| Change in urine color from red to dark cherry hue | Tearing | With an extensive burn, damage to the bone structure may occur. |
| Diarrhea | Pain in the eyes | Redness of the skin around the main wound |
| Difficulty breathing, shortness of breath | Pain behind the sternum | Tissue necrosis |
| Blue coloration of the skin | A sore throat | Decreased sensitivity |
| Darkening of lips and strong breath | Laryngospasm | Formation of blisters filled with cloudy liquid on the affected areas of the skin |
| The appearance of red-brown spots on the teeth | Laryngeal edema | |
| Heart failure | Pulmonary edema |
IMPORTANT! In most cases, when it comes to poisoning with sulfuric acid or its vapors, the life of the victim is under real threat.
Emergency measures
It is better to rinse burned eyes and skin with water than not to rinse at all.
In any situation where sulfuric acid has entered the esophagus, eyes, skin or respiratory tract, calling or personally delivering the victim to the hospital is mandatory. And as quickly as possible.
IMPORTANT! Battery acid poisoning requires the same actions as H2SO4 poisoning, since most devices use sulfuric acid compounds.
While waiting for medical help, you can try to relieve symptoms and prevent further toxicity using home remedies.
If sulfuric acid is ingested, you must:
- Place the victim on his right side in a comfortable position.
- Provide peace and fresh air.
- It is allowed to give foods rich in protein - egg whites, milk; It is also possible to swallow a small amount of rice water or cooled rice porridge. But in very small quantities so as not to stretch the stomach.
- If you suspect or have obvious symptoms of gastric bleeding, apply a container or ice pack to your stomach.
- In case of critical condition, resuscitation measures must be started.
ATTENTION! Any other actions during intoxication caused by sulfuric acid are prohibited.
It is strictly forbidden to:
- flush the stomach;
- provoke vomiting;
- give drink;
- try to neutralize the acid with other chemical compounds;
- give any medications, with the exception of cardiac medications, if they are vitally necessary;
- use traditional medicine.
IMPORTANT! Sulfuric acid is one of the most aggressive compounds, and the slightest mistake in providing first aid can cause a sharp deterioration in the condition. This is especially true for vomiting and gastric lavage: if previously it was believed that eliminating acid from the gastrointestinal tract helps, recent studies have shown: during the return passage through the esophagus, acid greatly injures mucous tissues, disrupts blood circulation, affects blood vessels, which can result in organ ruptures and major blood vessels, and, as a result, extensive internal bleeding.
VERY IMPORTANT! This provision regarding gastrointestinal lavage and vomiting is relevant not only for sulfuric acid, but also for all other acidic compounds. Also, you should not use baking soda and other alkalis to neutralize acids, since together these substances significantly increase gas formation, and this, in turn, can also result in rupture of internal organs and massive bleeding.
If sulfuric acid comes into contact with eyes or skin:
- It is necessary to rinse the affected areas as quickly as possible. The optimal liquid for this is physiological saline solution or so-called “water for injection”. If such means are not at hand, you can use large quantities of running water. Even if concentrated sulfuric acid gets in, for example, from a car battery, which enters into a violent chemical reaction with water, it is better to rinse at least with it than not to rinse at all. Still mineral water from a bottle is also suitable.
- Cover the damage with a clean handkerchief, cloth or napkin to prevent dust and dirt from getting on the burned areas, which will further worsen the situation.
If you inhale sulfuric acid fumes:
- Provide the victim with a flow of fresh air: open all doors and windows, take him outside, to the balcony, move him to the window.
- Give milk or Borjomi-type mineral water to drink.
- In critical condition, begin cardiopulmonary resuscitation.
A doctor is always needed, even if the burns are minor, and the victim is conscious and feels well.
Inpatient therapy
It is in the hospital that the patient will receive professional treatment aimed at reducing pain, restoring the functions of the affected organs and generally improving their condition. Depending on the severity of the poisoning, the doctor may make the following prescriptions:
- gastric lavage through a tube;
- droppers with solutions;
- hemostatic drugs;
- antibiotics to prevent inflammation;
- painkillers to avoid painful shock;
- oxygen mask;
- auxiliary therapy to maintain and restore the functioning of the liver, heart and kidneys;
- in cases of severe poisoning - mechanical ventilation or cardiopulmonary resuscitation;
- a diet based on fasting in the first 2 days, then - nutrition approved by the doctor depending on the degree of organ damage.
Hydrochloric acid: clinical signs, emergency care
This compound with hydrogen chloride is a colorless, caustic liquid with a very characteristic odor. The acid is so strong that it can dissolve many metals. It is used in the leather industry, the production of glue, alcohols, and other acids, and is found in textiles, hydrometallurgy and pharmaceuticals.
IMPORTANT! In a percentage of 0.3–0.5, hydrochloric acid is present in gastric juice, facilitating the digestion of food and protecting the human body from opportunistic microorganisms. For a lethal dose taken orally, only 15 ml of concentrated liquid is enough.
The most common poisoning is HCL acid vapor. The most dangerous concentration is 24–38%, and in this regard, the circulation and use of a substance of such saturation is strictly limited, since the greatest danger is posed by toxic mists formed by the combination of hydrochloric acid vapor and air.
Symptoms of HCL poisoning
Hydrochloric acid is often found in batteries
The clinical picture of hydrochloric acid poisoning is in many ways similar to damage caused by other acids and also requires a mandatory medical examination, regardless of the severity of the damage and the condition of the victim.
|
|
|
| Redness of the skin | Acute pain in the mouth, esophagus and stomach | Pain, feeling of burning or “sand” in the eyes |
| Formation of small blisters with thin walls filled with cloudy liquid mixed with blood | Vomiting with blood and bile | Excessive lacrimation and photophobia, redness of the conjunctiva |
| Skin acquires a dirty yellow tint | Internal bleeding | Sneezing, coughing, nausea |
| Sharp intense pain | Feeling of suffocation | Swelling of the airways and difficulty breathing |
| A burning sensation in the affected area | Development of laryngeal edema | Pain and soreness in the nasopharynx, hoarseness of voice |
| Painful shock in extensive and deep burns | Oral burn accompanied by blackening of soft tissues | Nasal discharge mixed with blood |
Chemical burn 3rd degree
IMPORTANT! It is possible to reliably assess the depth and severity of skin burns with this acid only 5–7 days after the initial injury. The fact is that this substance immediately begins to corrode the tissue and even after thoroughly washing the surface of the affected areas, a certain amount of acid remains, which continues to further injure the skin layers. In severe cases, it comes to tendons, muscles and bone structures.
First aid for hydrochloric acid intoxication
Saline or Ringer's solution is the best solution for cleaning acid burns.
All variants of hydrochloric acid poisoning are a direct threat to the life and health of the victim. Therefore, calling doctors or independently transporting a poisoned person to a hospital should be urgent.
In cases where it is not possible to quickly transport doctors to a patient or a patient to a hospital, you need to act independently while waiting for professional help.
If hydrochloric acid is swallowed:
- rinse your mouth with water or baking soda solution without swallowing the liquid;
- take egg white or milk orally in small quantities to avoid stomach distension and vomiting;
- help the victim take a comfortable position on his side and ensure peace;
- Closely monitor the condition of the poisoned person and, in case of loss of consciousness, lack of pulse or breathing, begin resuscitation.
And again: inducing vomiting or organizing gastric lavage in case of acid poisoning is strictly prohibited!
If there has been poisoning with hydrochloric acid vapors:
- eliminate contact with toxic fog as quickly as possible: organize ventilation, remove or carry the victim out of the contaminated room, put a respirator or gas mask on the patient;
- rinse your mouth with water, milk or baking soda solution;
- If possible, give warm milk and other protein-rich foods to drink;
- ensure rest in a lateral position;
- in case of critical condition, begin artificial heart massage and breathing stimulation.
IMPORTANT! If the victim’s condition does not cause concern, and he is able to talk, breathe and walk, this does not mean that everything is in order. A sharp deterioration can occur at any time, so it is always necessary to monitor the victim’s pulse and breathing rate at regular intervals of 10–15 minutes.
In case of skin damage:
- rinse the affected area with plenty of running water and saline;
- rinse skin with soap and water;
- cover the burn with a clean cloth or apply a gauze bandage.
ATTENTION! Under no circumstances should you smear a burn after hydrochloric or other acid with any anti-burn agents, creams, oils, cosmetic products, herbal infusions, alcohol and other substances.
How can you get poisoned by alkalis?
Alkalis are used in industry, medicine and in everyday life (ammonia, slaked lime, caustic soda and others). Poisoning by alkalis occurs when emergency situations occur at work, as well as when safety precautions are violated when working with alkalis.
At home, poisoning occurs through accidental ingestion, as well as for the purpose of suicide.
Alkalis have a strong damaging effect on:
- The mucous membrane of the gastrointestinal tract (burned, tissues become loose);
- Protein structures (proteins dissolve);
- Upper respiratory tract (in case of intoxication with ammonia, lime);
- Kidneys (remove alkalis from the body);
- Intestines (alkali is removed from feces).
Symptoms of poisoning
Alkalis have a burning effect to a greater extent than acids. In this regard, the clinical picture of poisoning is as follows :
- Burn of the skin around the oral cavity and mucous membrane. There is redness, swelling and severe pain;
- Thirst;
- Severe pain syndrome. Pain is noted in the chest and epigastrium. She is often intolerable;
- Bloody vomiting due to the formation of blood vessels in the deep layers of tissue;
- Perforation of the esophagus. The stomach rarely suffers, since a larger volume of alkalis is neutralized in it;
- Asphyxia by vomit or disintegrated tissue of the esophagus;
- Diarrhea mixed with blood;
- Shock;
- Stopping breathing and heartbeat (in severe cases).
Since the victim experiences severe pain, pain shock develops . In the first hours after poisoning, death may occur due to a state of shock (pain, burns).
- A characteristic burn of the skin around the oral cavity when acids penetrate through the mouth;
- Characteristic changes in the mucous membrane of the oral cavity and esophagus. Its color and structure change;
- Severe pain that worsens during swallowing and vomiting;
- Swelling of the esophagus, which interferes with swallowing and breathing;
Nitric acid: symptoms, first aid
At a concentration above 85%, nitric acid begins to smoke
HNO3 is a colorless liquid with a pungent odor, used to create chemical fertilizers, used in metallurgy, printing and dairy industries. Poisoning with nitric acid most often occurs in the form of burns on the skin when, for some reason, there was short-term or long-term contact.
In most cases, we are talking about industrial intoxications and non-compliance with safety rules when using nitrogen fertilizers in domestic conditions.
Signs of HNO3 poisoning
|
|
|
| Dizziness | Acute pain, pain in the oropharynx, esophagus, stomach | Redness followed by a change in skin color to a greenish-yellow hue |
| Cough | Intense nausea, vomiting with blood clots | Intense pain at the site of contact with acid |
| Laryngospasms | Heart rhythm disturbance | The appearance of a rash in the form of blisters of various sizes filled with serous fluid |
| Labored breathing | Pink or reddish urine | Swelling and tissue necrosis |
| Burns of the nasal and oral cavity | Yellow discoloration of the mouth and tongue | After a few hours, the burn area begins to form a dense crust. |
| In severe cases, pulmonary edema | Difficulty swallowing | In case of severe burn – blackening of the skin |
| Stunned state | Bloating | |
| Nausea, sometimes vomiting | Loss of consciousness | |
| Impaired coordination and consciousness | Decreased body temperature |
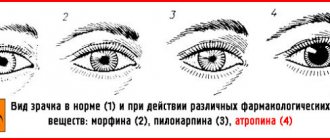
ATTENTION! The most dangerous and difficult to treat variant of nitric acid poisoning is eye burns. Swelling and spasms of the eyelids, severe pain, decreased visual perception, lacrimation, discoloration of the conjunctiva and cornea - all these are symptoms of acid damage.
Urgent actions
If the pain and burning does not stop, you need to wash the burn again and again.
The faster and more competently first aid is provided for nitric acid intoxication, the greater the chance of saving the victim not only life, but also health. Therefore, it is necessary first of all to immediately call doctors, informing the dispatcher about the nature and location of the burn.
If HNO3 enters the esophagus:
- do not induce vomiting;
- rinse your mouth with milk, water or baking soda diluted in water without swallowing;
- provide comfort, warmth and fresh air;
- if the victim loses consciousness, place him in a stable position on his side;
- control pulse and breathing;
- If critical signs (lack of pulse and breathing) appear, immediately begin resuscitation measures.
If you inhale nitric acid vapors:
- exclude further contact with toxic air: remove, take the poisoned person outside, to another room, open all accessible windows and doors;
- loosen items of clothing or work equipment that impede free breathing;
- if the victim is conscious, rinse your mouth with water and soda or milk;
- can be given as a drink, if the swallowing function is not impaired, milk, alkaline mineral water without gas;
- when critical symptoms appear, begin artificial respiration and chest compressions and do not stop until signs of life return or doctors arrive.
IMPORTANT! The danger of injury from acids also lies in the fact that 30-60 minutes after poisoning the victim may feel quite well: talk, walk, drink. But the probability that within 4–6 hours a deterioration will occur, leading to pulmonary edema and subsequent death, tends to 90%. Therefore, medical attention is needed in any case, even with minor burns.
Milk will somewhat soften burned mucous membranes
For skin burns with nitric acid:
- remove and cut off items of clothing soaked in acid;
- rinse the affected area with running water for 15–20 minutes (if severe pain and signs of acid on the skin remain, rinsing should be continued for another 10–20 minutes);
- treat damaged areas with soapy water or baking soda diluted in water;
- cover burns with a clean cloth or apply a loose gauze bandage;
- in case of severe burns combined with inhalation of vapors, be prepared to begin resuscitation efforts.
ATTENTION! Under no circumstances should you apply cotton wool or paper napkins to the burn site, or wipe the damaged skin with ice, ointments, healing compounds, alcohol or any other improvised or medicinal means.
Consequences
Chemicals cause vocal cord burns and muteness. The effect of these elements on the esophagus is acute pain and scar tissue that makes swallowing difficult.
Contact of acid or alkaline solutions with the mucous membrane of the eye can result in loss of vision. Toxic effects on the liver contribute to the development of jaundice. The consequences of acid or alkaline poisoning can be very severe, including disability and death of the patient.
Oxalic acid: clinical signs, emergency care
Beekeepers often become victims of this acid.
This acid is naturally found in sorrel and rhubarb; within acceptable levels of 0.2 mg/l it can be present in fresh water bodies. Oxalic acid is mainly produced artificially and is used to make various types of impregnations for wood and metals. It is also found in the leather craft and pulp industry.
Typically, intoxication occurs when the substance is ingested. Poisoning with oxalic acid occurs much less frequently than intoxication with other aggressive chemicals, but only it is dangerous due to a long delayed effect.
The fact is that some derivatives of this acid are extremely poorly soluble, so when they enter the human body they settle in the kidney structures in the form of various types of stones.
Symptoms of poisoning
Signs of intoxication with oxalic acid can be divided into three groups according to the severity of the condition and the rate of development of pathologies:
| Lightning form | Acute form | Subacute form |
|
|
|
IMPORTANT! Unlike other acids, oxalic acid almost never provokes serious cicatricial changes in the gastrointestinal tract, being limited only to superficial damage. However, this does not mean that intoxication with oxalic acid is less dangerous than with other similar substances.
First aid
Milk can be used as a mouthwash
The lethal dose of this acid for an adult is only 15–20 g, and 5 g is enough for a child. Therefore, if intoxication with this substance is suspected, the victim must be taken to doctors as quickly as possible.
On your own, if swallowed, you can:
- rinse your mouth with unboiled milk or plain water without swallowing;
- provide fresh air and a comfortable position to the victim;
- place a container of ice on your stomach;
- give the victim ice cubes to swallow;
- in case of loss of consciousness, pulse and breathing, begin resuscitation.
Burns to the eyes or skin are much less common, but also require medical attention. While waiting for doctors, you must:
- rinse the affected areas of the skin or eyes with plenty of running water, preferably for 10–20 minutes;
- without wiping, cover the skin or eyes with a clean cloth or gauze;
- sit or lay the victim in a comfortable position.
IMPORTANT! Any other actions, in particular the administration of calcium gluconate, which destroys oxalic acid, should be performed only by medical professionals. The price of independent attempts to neutralize is a pronounced deterioration in the victim’s well-being and the risk of additional problems.
Why is intoxication dangerous?
Hydrochloric acid poses a particular danger to the human body. In case of poisoning with such a substance, serious complications and disturbances in the functionality of the body may develop.
Complications:
- Impaired liver function, as a result of toxic hepatitis,
- Bleeding in the stomach due to destroyed walls of the organ,
- Shock from pain when acid hits a large area,
- If it gets in the eyes, visual impairment may occur,
- Serious problems with the kidneys,
- Impaired breathing, suffocation, lack of air,
- Development of a coma.
Such consequences develop gradually depending on the degree of poisoning.
Ascorbic acid: signs of overdose, first aid
Neither adults nor children should eat ascorbic acid with glucose more than 3 tablets per day
Beloved by both children and adults, “vitamin C” regulates a wide variety of processes in the human body: from blood clotting to the synthesis of certain mineral compounds. It is involved in metabolism, helps the immune system fight viruses and bacteria, has a positive effect on the central nervous system and strengthens the skin.
However, such a useful compound can also be dangerous if you overdo it and exceed the permissible dosage. Unfortunately, the vast majority of cases occur in children. Parents, faced with incomprehensible symptoms, do not even think about whether it is possible to be poisoned by ascorbic acid, and look for other culprits.
Signs of ascorbic acid intoxication
Such poisoning can occur for three main reasons:
- accidental ingestion of large amounts of vitamins;
- non-compliance with the dosage during a course of treatment in tablet form or in the form of injections;
- simultaneous use of medicinal vitamin C with foods rich in this substance.
Ascorbic acid poisoning manifests itself in the form of the following symptoms:
- sleep disorders;
- increased sweating;
- irritability, feeling of constant anxiety or restlessness;
- flatulence, bloating;
- nausea;
- bowel dysfunction;
- cramps in the stomach.
Unpleasant nausea
If a large dose of ascorbic acid enters the gastrointestinal tract at the same time, intense vomiting may begin.
ATTENTION! In children, intoxication with this acid can also manifest itself in conjunction with the main symptoms in the form of skin rashes, which are accompanied by noticeable redness, itching, sometimes burning and mild pain.
Unfortunately, these are not all the negative consequences of ascorbic acid poisoning. She is capable of:
- affect the mucous membranes of the stomach, provoking the beginnings of a peptic ulcer;
- lead to slow absorption of certain groups of vitamins;
- cause subsequent allergies to vitamin C in any form;
- disrupt the functioning of the kidneys and urinary system;
- reduce the performance of the pancreas;
- affect the hematopoietic system.
And intoxication of pregnant women with this substance can result in severe metabolic disorders and a congenital allergy of the unborn child to ascorbic acid.
First aid
Drinking plenty of fluids is the first aid for intoxication with ascorbic acid (but not other acids!) Ascorbic acid is the only one of its kind that is perfectly soluble in water and does not damage the gastrointestinal tract when vomiting.
IMPORTANT! A lethal single dose ranges from 20 to 30 g. This amount is approximately equal to one daily dose for an adult.
What to do if you are poisoned by “vitamin C”?
- In case of a dosage error or accidental ingestion of less than 15–20 g of ascorbic acid, you should drink as much fluid as possible.
- When a dose of more than 15 g is taken at a time, you need to drink 1-2 glasses of water and organize gastric lavage.
- Take any enterosorbents.
And be sure to call a doctor or go to the nearest hospital yourself. Even with a slight excess of the dosage of ascorbic acid, pronounced disturbances in well-being and serious changes in the body as a whole can be observed.
Receipt
In laboratory conditions, hydrogen chloride is obtained by reacting concentrated sulfuric acid with sodium chloride (table salt) with low heating:
NaCl(solid) + H2SO4(conc.) = NaHSO4 + HCl
HCl can also be prepared by hydrolysis of covalent halides such as phosphoryl chloride, thionyl chloride (SOCl2), and hydrolysis of carboxylic acid chlorides:
PCl5 + H2O → POCl3 + 2HCl R-COCl + H-OH → R-COOH + HCl H2O + O=SCl2 → SO2 + 2HCl
In industry, hydrogen chloride was previously obtained mainly by the sulfate method (Leblanc method), based on the interaction of sodium chloride with concentrated sulfuric acid. Currently, direct synthesis from simple substances is usually used to obtain hydrogen chloride:
H2 + Cl2 ⇌ 2HCl
Under industrial conditions, synthesis is carried out in special installations in which hydrogen continuously burns with an even flame in a stream of chlorine, mixing with it directly in a burner torch. This ensures a calm (without explosion) reaction. Hydrogen is supplied in excess (5 - 10%), which makes it possible to completely use the more valuable chlorine and obtain hydrochloric acid uncontaminated with chlorine.
Hydrochloric acid is prepared by dissolving hydrogen chloride gas in water.
Acetic acid: symptoms, urgent actions
Variety of forms and types of acetic acid for home use
This substance is found in almost every home in the form of a 9% solution or 70% vinegar essence. A transparent liquid with a sharp characteristic odor and a strong sour taste is used for preparing marinades, brines, and seasonings; used in cooking as additives for “sourness”, and is also involved in the production of medicines.
Statistics. Most cases of suicidal acid poisoning involve vinegar.
Clinical signs of acetic acid intoxication
When people talk about vinegar poisoning, they most often mean the entry of a caustic substance into the gastrointestinal tract.
Symptoms can be divided into stages:
| First (mild) degree | Second (middle) degree | Third (severe) degree |
|
|
|
ATTENTION! The second and third degrees of poisoning are accompanied by severe blood thickening and renal failure. As symptoms develop, one stage changes to another, and the final outcome of the action of acetic acid is unpredictable.
Urgent measures
It is necessary to check the pulse and breathing strength regularly until help arrives or the person regains consciousness
Like poisoning with carbon dioxide and other toxic compounds of this type, intoxication with vinegar requires immediate medical supervision. Adequate and complete assistance is possible only in a hospital setting.
There are only a few things you can do on your own:
- rinse your mouth thoroughly and repeatedly with water, avoiding swallowing the liquid;
- provide a comfortable position for the victim, preferably lying on the right side;
- ventilate the room;
- in case of painful shock and loss of consciousness, monitor the pulse and breathing rate of the poisoned person;
- When a sign of a critical condition appears, begin resuscitation procedures.
It is strictly forbidden to:
- induce vomiting and flush the stomach yourself;
- give drink, food or any medications;
- try to neutralize the acid with soda, soap solution or other alkalis;
- warm, massage the stomach.
ATTENTION! If vinegar essence gets on the skin or eyes, it is necessary to thoroughly rinse the affected areas with running water for 15–30 minutes. And be sure to show the burn site to the doctor.
Possible complications
Complications depend on the severity of the burn. The outcome is favorable for grades 1 and 2.
In severe cases, infections develop, resulting in tissue death. The functioning of internal organs: heart, digestion, liver, kidneys, central nervous system may be impaired for life. The person may remain disabled. To prevent consequences, it is necessary to diagnose the condition in a timely manner and provide first aid to the victim.
Prevention is important. Preventive measures to avoid disease include proper storage, transportation and handling of aggressive substances. At home, hazardous substances and chlorine-containing cleaning products should be stored in hard-to-reach places, tightly closed, in labeled containers. Prevention measures are especially important if there is a small child in the house.
source
Lemon acid
Citric acid is added to compotes and fruit drinks, marinades and first courses, desserts and baked goods.
This compound in the form of powder or granules is often used in the household during cooking, canning, pickling, and is used to clean the kettle and other utensils from scale. Another popular way to operate this compound is “pop”.
Most people don’t even think about whether it is possible to be poisoned by citric acid, and without fear they add it to dishes, boil it in a kettle and keep bags with a whitish-yellow substance freely available to children.
Signs of citric acid poisoning
Intoxication with this substance is possible if a large amount enters the digestive tract at once. Vapor poisoning is extremely rare.
As a rule, symptoms appear immediately or within half an hour after ingesting citric acid in the form of:
- dizziness and severe weakness;
- intense pain in the mouth, stomach;
- headache;
- severe nausea;
- vomiting with red or black impurities;
- loose stools that are bloody or black in color;
- pale, sometimes bluish skin;
- lack of air, shortness of breath;
- rapid heartbeat;
- lowering blood pressure;
- muscle cramps.
In a serious condition, the victim may lose consciousness, which may well end in coma and even death.
ATTENTION! The main danger of poisoning with this acid is the high risk of internal bleeding, which is manifested by red-black vomit and similar-colored stools. Pale and blue skin is a sign of blood loss.
In rare cases, burns may occur upon contact with concentrated citric acid and skin. In this case, swelling with redness forms at the site of injury, sometimes bubbles with cloudy liquid appear, which is accompanied by pain and burning.
IMPORTANT! People with cardiac dysfunction, in particular those suffering from ischemia, can suffer an acute heart attack when poisoned with citric acid.
First aid
You can fill a regular heating pad with ice or ice water.
The initial action is to call an ambulance. It is advisable to tell the dispatcher not just about poisoning, but specifically name the cause of intoxication. Also indicate the age of the victim and the approximate time that has passed since the poison was taken.
You can do it yourself:
- place the victim in a comfortable position;
- in the absence of consciousness, place the poisoned person on the right side in a stable position;
- rinse your mouth repeatedly with plain water without swallowing it;
- provide fresh air;
- Place a container or ice pack on your stomach, which will reduce the rate of absorption of the poison into the gastrointestinal tract.
You cannot drink, eat, take any medications or folk remedies, or heat or massage your stomach.
IMPORTANT! It is strictly forbidden to provoke vomiting and rinse the stomach!
If citric acid gets into your eyes or skin, rinse these areas with plenty of running water and cover with a clean gauze pad or other cloth without wiping.
Treatment
If necessary, hemostatic drugs and glucocorticosteroids can be used as emergency aid; in case of shock, parenteral administration of dopamine or adrenaline is practiced. Artificial respiratory support is necessary when alkali vapor poisoning occurs.
At the next stages of therapy, the patient is prescribed diuretics to accelerate the evacuation of chemicals from the body. Treatment regimens may include hepatoprotectors, non-steroidal anti-inflammatory drugs, and analgesics.
In case of alkali poisoning, antibiotics must be used. They are necessary to prevent the development of bacterial or fungal infections.
Prevention
Everything within a child's reach must be safe
The main safety measure is basic caution:
- Do not work with pesticides, acids, any caustic substances or their vapors without protective equipment both at home and at work.
- Store acids out of the reach of children in airtight and tightly sealed containers.
- When preparing food, carefully measure the required amount of acetic or citric acid, and if you use them as a cleaning agent, notify your household of your actions.
- Carry out vehicle inspections in a timely manner and check the health of the battery.
- Never taste glue, varnishes, paints, garden chemicals and fertilizers, or any potentially hazardous substances or liquids.
- Teach children safety rules.
Don't leave kids unattended
Acid poisoning carries a real, not theoretical, danger to human health and life. Even if everything went well, the victim’s condition does not inspire fear, and the symptoms are mild; no one is immune from complications and delayed side effects of intoxication.
Antidotes and their use
After the incident, it is important to correctly assess the patient’s condition and administer antidotes:
- a good antidote to vinegar is Almagel, lime water or burnt magnesia;
- The pathogenesis of acid poisoning involves the clotting (hemolysis) of red blood cells in the blood. The intravenous administration of 4% sodium bicarbonate solution, glucose solution, and saline solutions will help stop the mechanism of crystallization of red blood cells;
- breathing spasms will be stopped by atropine, irrigate the throat with prednisolone, hydrocortisone;
- To treat acidosis (blue discoloration), intravenous administration of Trisamine is recommended.
- corticosteroids are used to stop shock.
Subsequent therapy after gastroscopy in the clinic is symptomatic. Painkillers and antibiotics are prescribed. Flushing blood with an artificial kidney (hemodialysis).
Literature
- Levinsky M.I., Mazanko A.F., Novikov I.N. “Hydrogen chloride and hydrochloric acid” M.: Chemistry 1985
To prevent inhalation poisoning with hydrochloric acid both at home and at work, a number of requirements must be observed:
- work in special clothing that covers all parts of the body;
- work only using personal protective equipment (goggles, respirator, gloves);
- In the room where concentrated acid is used, ensure effective ventilation.
Concept
Hydrochloric acid belongs to the group of highly caustic and toxic substances.
The second name of the substance is hydrogen chloride.
It is a clear liquid (sometimes a yellowish color may be present). The smell from it is pungent and unpleasant. Sometimes you can see a cloud of smoke above the container with it.
Hydrochloric acid vapors are no less toxic and have the same destructive effect on the body.
This substance is obtained industrially - hydrogen chloride (gas) is dissolved in water. ()