Isopropyl alcohol is one of the simplest monohydric alcohols. It belongs to the aliphatic series, that is, it has no aromatic bonds. At the same time, the smell of the substance is pungent. Looks like ethanol. It is used as a substitute in various areas of life: cosmetics, household chemicals, perfumes. Its use as a hand disinfectant is very popular, as it provides better disinfection compared to ethyl alcohol.
Isopropanol is also used in medicine in the treatment of otitis externa and dermatitis. In the latter case, treatment is carried out by rubbing the affected areas of the skin. There is almost no harmful effect. It requires great care when handling as it is flammable and toxic. It is regulated by GOST 9805–84, which describes different types of this alcohol: absolute and technical. Instructions for their use are accordingly different.
It is often confused with isooctyl alcohol , but these are two different substances. Isopropyl is a lower alcohol. Structurally, it is closest to ethanol. A common misnomer is propylene alcohol.
Isopropanol is often confused with isopropyl chloride, which is formed from propylene and hydrogen chloride.
Isopropanol
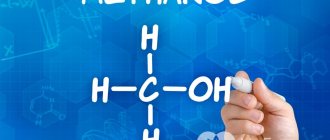
Isopropanol, IPA is a substance of organic nature. The structure of the 1-propanol isomer has the same qualitative and quantitative composition, but differs in the order of connection of atoms. Isopropyl alcohol is represented by the following formula: CH3CH(OH)CH3. This composition was obtained as a result of chemical experiments with propanol. This compound is classified as a substance of the third hazard class.
The production of isopropyl alcohol must occur strictly in accordance with GOST 9805 84, which ensures compliance with physical and chemical characteristics. Only compliance with the conditions of GOST 9805 84 can ensure the required quality standard and the corresponding manufacturer’s product passport. Isopropyl is a technical alcohol, which is strictly prohibited to drink.
Occupational safety[ | ]
Amyl alcohol is a toxic substance[2]. Its maximum permissible concentration in the air of the working area[3] is 10 mg/m3 (amyl alcohol, maximum single dose) and 5 mg/m3 (isoamyl alcohol, maximum single dose). And the value of the odor perception threshold can exceed 300 mg/m3[4]. Moreover, for some people it can be significantly higher than the average value. For this reason, it can be expected that the use of widely used filtering RPE in combination with “replacing filters when an odor appears under the mask” (as is almost always recommended in the Russian Federation by suppliers) will lead to excessive exposure to harmful vapors for at least some workers - due to late replacement of gas mask filters. Therefore, to protect against amyl alcohols, more effective technology changes and collective protective equipment should be used.
Chemical properties
In the presence of oxidizing agents (chromic acid or copper), alcohol easily turns into acetone. If you heat the substance together with sulfuric acid, you can get propyl alcohol. Isopropyl alcohol is a strong solvent and can damage rubber products. All essential oils and resinous substances dissolve well in it. When the compound burns, water and carbon dioxide are formed without soot or smoke.
Isopropylene mixes well with water; the freezing point of the resulting liquid depends on the proportions. The chemical compound has a pronounced alcoholic odor, which is much more intense than that of ethanol. Drug intoxication is also more pronounced when consuming this type of alcohol.
Precautionary measures
Like any chemical compound, IPA is a dangerous substance that requires careful handling. It is necessary to use alcohol in a well-ventilated area, since an excess of its vapors and entry into the body can lead to intoxication. It is necessary to store IPS away from fire and direct sunlight, in places inaccessible to children.
Alcohol does not have the ability to be absorbed into the skin, but with regular interaction it can cause a chemical burn. It is strictly forbidden to allow substances to come into contact with mucous membranes.
Receipt
Isopropyl alcohol can be made by adding a hydrogen molecule to acetone. Another method involves mixing propylene with sulfuric acid and then heating it. Oxidation of paraffin with air is less commonly used. The following types of alcohol are used in industry:
- rectified (the most purified);
- technical (contains more harmful impurities).
Chemical companies produce tons of this product, since it is difficult to find an industry where it is not used. The product can be purchased wholesale and retail; isopropyl alcohol is sold at a very affordable price in liter containers. If a liter of product is not enough, a larger volume can be ordered from the online store. Absolute isopropyl alcohol has a higher price compared to technical alcohol, as it is classified as an ultra-pure chemical substance.
Where is it sold?
The easiest way is to buy the product in bulk from the manufacturer or direct representative. The price depends on the degree of purity and type of packaging and is 80-200 rubles per 1 kg.
We recommend: “Mole” for cleaning pipes: how to use it so as not to harm yourself or the pipes?
The most common forms of containers:
- bottles with a capacity of 0.5 or 1 l;
- canisters with a volume of 3–30 l;
- barrels of 200 l.
At retail, the product can be purchased in small quantities for household purposes in specialized chemical stores.
It is theoretically possible to find isopropanol in pharmacies, but now this product is very rare. In addition, alcohol is available by prescription to prevent misuse by alcohol dependent individuals.
When purchasing, it is better to choose absolute alcohol, which can be used for an unlimited number of everyday household needs. Even for technical purposes, preparing homemade detergents and antifreeze agents, a pure product without foreign impurities is preferable.
Application of isopropanol
The areas of application of isopropyl alcohol are different; it is used very actively in the national economy. The exception is the food industry, since drinking isopropyl alcohol is very harmful to health even in minimal doses.
Medicine
In medicine, isopropanol is used as a universal disinfectant. It is included in the impregnating liquids for medical wipes and is used to wipe the injection site. The ethyl alcohol solution previously used for these purposes is used much less frequently.
Biology
IGS is widely used as a preservative for storing tests and genetic material. It is much less toxic than formaldehyde (a derivative of ammonia), which was previously actively used for these purposes.
Most insect control products contain IPA and should be used with caution. Car windows will be reliably protected from icing, and their optical qualities will be significantly improved by using special cans containing isopropyl alcohol.
Other industries
The price of isopropyl alcohol is low, which allows it to be included in the product without economic damage. It is less toxic than methanol, which allows the compound to be added to cosmetics and perfumes. Since such products have contact with the skin and are absorbed through its pores, their production requires absolute alcohol, that is, completely free of harmful impurities.
Paint and varnish production requires IPA, as this product is an excellent solvent. The production of antifreeze cannot occur without such alcohol. This liquid owes its anti-freezing properties to it. The table shows that the higher the concentration of isopropanol in the anti-freeze product, the lower temperatures it tolerates.
As a solvent, alcohol is common for use in electronics for cleaning parts. In the household, the substance can be used to successfully clean surfaces and fabrics. It should be used very carefully on synthetic surfaces, especially vinyl and rubber.
Isopropyl alcohol on the domestic market
Currently, there are two isopropane producers in Russia:
- Orsk synthetic alcohol plant. This company produces isopropane from propylene. The advantage of its products is the absence of a strong odor. Buyers are offered two types of isopropane - absolute (97%) and technical (87%). They are mainly used for the manufacture of perfumes and honey. solutions and windshield washes. The plant uses outdated sulfuric acid production technology.
- Dzerzhinsky. Isopropane (technical 94–98% and absolute 99.7–99.95%) is produced from acetone using modern technology. It has better color and also has a characteristic odor without foreign impurities. The main application of the products of this plant is in the paint and varnish industry; it is also used for the production of antifreeze liquids. The main difficulty that Synthesis Acetone faces is unstable prices for raw materials.
How can you get poisoned?
You can become poisoned by isopropyl alcohol by inhaling its vapors or accidentally ingesting it. In some cases, low-quality vodka or moonshine may contain a certain amount of such alcohol. A toxic dose for humans is 15 grams of the substance. Smaller amounts may cause digestive upset such as diarrhea. Some antisocial individuals specifically use liquids containing isopropanol to obtain drug intoxication.
Symptoms
Pathological manifestations of poisoning are associated with the damaging effect of acetone, which is formed when the substance is broken down by alcohol dehydrogenase. This liver enzyme is involved in the inactivation of many toxic substances entering the body.
- At first, heaviness and pain appear in the head, which is accompanied by dizziness and unsteadiness of gait, problems with coordination appear, and speech becomes incoherent. All this resembles the clinical picture of alcohol intoxication, but a pronounced smell of acetone appears from the mouth.
- Subsequently, drowsiness sets in, causing the poisoned person to fall asleep. Intoxication can cause visual impairment in the form of blurred images of objects and double vision. Flashing dots and dark spots appear before the eyes.
- Next, severe pain develops in the muscles of the limbs, which may be accompanied by tissue necrosis. There are unpleasant sensations in the abdomen, lower back, and chest. In case of severe poisoning, respiratory failure may develop with a disorder in the function of the respiratory center, which is located in the brain stem. Cardiac activity also weakens, blood pressure decreases or increases, and pulse filling decreases. Convulsive syndrome may develop.
A dose of 250 grams is lethal for humans.
First aid
In case of isopropanol poisoning, the victim must be hospitalized immediately. To remove any remaining toxic substance, rinse the stomach with sodium bicarbonate solution and induce vomiting. A cleansing enema will remove any remaining substance from the intestines. If consciousness is preserved, the poisoned person should be given any enterosorbent agent:
Alt: Smecta Title: Drug Smecta
- Enteros-gel;
- Polysorb;
- Smecta.
Diagnostics
Isopropanol poisoning is established on the basis of anamnesis, clinical picture and the results of a biochemical blood test and a clinical urine test for the content of the toxic substance.
Treatment
Treatment is aimed at restoring affected vital functions and removing poison from the body.
Stabilization of well-being
In order to stabilize the activity of the affected organs, a solution of glucose with ascorbic acid and vitamin PP is administered. Intravenous administration of saline and sodium bicarbonate will help restore the disturbed acid-base balance. In severe cases, hemodialysis (artificial kidney) is necessary to remove toxic substances from the body.
Eliminating Poison
To eliminate poison from the body, use:
- diuretics;
- laxatives;
- enterosorbents;
- cleansing enemas.
More intensive interventions include blood and plasma replacement fluid transfusions, as well as hemodialysis.
Specific neutralization of the poison is possible with the help of an antidote.
Antidote
Ethanol is an antidote for removing isopropyl alcohol from the body.
Symptomatic treatment
To eliminate pathological symptoms the following can be used:
- antihypertensive drugs;
- steroid hormonal drugs;
- respiratory analeptics;
- caffeine.
Severe pain may require the prescription of painkillers.
Impact on humans
The substance does not accumulate in the body. When consumed orally, it can cause intoxication similar to alcohol, and its intensity is 10 times higher than that of alcohol. Therefore, a dosage of 50 ml, which is considered highly toxic, can replace more than a liter of vodka. In general, it is difficult to be poisoned to the point of death, since a person falls into an alcoholic trance much earlier - unless, of course, he drinks at least 500 ml at a time. It is eliminated from the body from 5 to 16 hours.
But it is not recommended to use it as a substitute for alcohol, since the toxicity is 3.5 times higher than ethyl alcohol. It's all about acetone, which is a metabolite of this substance.
Isopropanol can also cause addiction , and it is often used by alcoholics who want a quick but good escape into oblivion. If a person’s concentration reaches 12 ppm, then within 4 hours he dies. According to GOST, isopropanol has toxicity class 3.
Large concentrations of vapors of this substance can cause headaches , irritation of the eyes and respiratory tract. However, this is not so easy to achieve, since you need to stay in an unventilated room for a long time. But if this continues for a long time, the person may lose consciousness. In practice, cases of severe isopropanol poisoning are rare.
Thus, isopropyl alcohol cannot be considered as a complete replacement for alcohol, although many substance abusers happily consume it internally. In industry and medicine, it has found its consumer, whom it has served faithfully for many years.
Camphor alcohol: application and pharmacological properties
It has the following pharmacological properties:
- antiseptic;
- anti-inflammatory and wound healing;
- is a local irritant;
- improves circulation processes in tissues;
- acts as a thinning agent on viscous secretions;
- has a cardiotonic effect.
Camphor alcohol has an excellent effect on the constriction of veins and blood vessels , improves blood microcirculation in the heart and brain vessels, expands respiratory functions, is a stimulant of the central nervous system, and accelerates metabolic processes in the heart muscle.
The camphor drug is excreted through the kidneys. In addition, camphor particles can even penetrate the respiratory tract and bile.
Notes[ | ]
- Amyl alcohols (Russian) / ed. I.L. Knunyants, N.S. Zefirov. - Moscow: Soviet Encyclopedia, 1988. - T. 1. Ablative materials - Darzan reaction. — ISBN 5-85270-008-8.
- Cheltsova M.A.
Amyl alcohols // Great Medical Encyclopedia: 30 volumes / chapter. ed. B.V. Petrovsky. — 3rd ed. - Moscow: Soviet Encyclopedia, 1974. - T. 1. A - Antibiosis. — 576 p. — 150,000 copies. - (Rospotrebnadzor).
No. 1695. Pentan-1-ol (Amyl alcohol); Pentan-2-ol (isoamyl alcohol) // GN 2.2.5.3532-18 “Maximum permissible concentrations (MPC) of harmful substances in the air of the working area” (Russian) / approved by A.Yu. Popova. - Moscow, 2020. - P. 117. - 170 p. — (Sanitary rules). - Pongsuriya Komthong, Shoichi Hayakawa, Tatsuo Katoh, Noriyuki Igura, Mitsuya Shimoda.
Determination of potent odorants in apple by headspace gas dilution analysis (English) //
Swiss Society of Food Science and Technology
LWT - Food Science and Technology (
Lebensmittel-Wissenschaft und - Technologie
). - Elsevier, 2006. - Vol. 39. - Iss. 5. - P. 472-478. — ISSN 0023-6438. - doi:10.1016/j.lwt.2005.03.003.