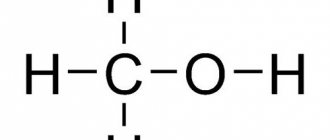Chlorine in the form of various compounds is present in all living organisms on the planet. A person needs it for the proper functioning of the digestive system and water metabolism, but its excess has a toxic effect and negatively affects health in general.
Chlorine poisoning requires mandatory medical supervision, and the task of the patient and his relatives is to help correctly and not make things worse until a doctor arrives. The information, photos and videos in this article, presented below, will help you navigate in case of danger and tell you about preventive measures.
Types and forms of clinical course and their symptoms
The severity of clinical manifestations depends on the degree of intoxication.
In case of mild poisoning, there is a burning sensation in the eyes, a sore throat, and acute rhinitis. Sometimes headaches and a subjective feeling of suffocation are noted. When examining the victim, salivation and lacrimation are detected. General condition is satisfactory. In case of moderate chlorine poisoning, a barking cough appears. Hoarseness, psychomotor agitation, vomiting, nausea, and pain in the stomach occur. Objectively: breathing is rapid, dry scattered wheezing is heard on auscultation. Body temperature is reduced, the pharynx is hyperemic and swollen.
Severe poisoning is characterized by the presence of all the symptoms that occur in mild and moderate cases, but with this degree of poisoning the symptoms are more pronounced. After some time, a period of remission begins, which lasts from 2 to 8 hours. During this period, the cough disappears and the victim calms down.
In case of fatal poisoning, clonic type convulsions occur. Involuntary urination and defecation occur. On examination, diffuse cyanosis, swelling of the neck veins, exophthalmos, convulsions of the limbs, and loss of consciousness are revealed. First aid with the use of bronchodilators does not relieve laryngospasm. Death occurs a few minutes later. The cause of death is progressive asphyxia and cardiopulmonary failure.
There are three degrees of poisoning with chlorine and chlorine-containing substances, which differ in the concentration of the substance and the time of its exposure to humans. There are mild, moderate and severe poisoning. Also, at very high concentrations of chlorine, poisoning occurs instantly, forming a lightning-fast form.
Symptoms of mild chlorine poisoning include:
- sore throat and nasopharynx;
- excessive lacrimation and rhinorrhea;
- redness of the eyes and mucous membranes of the mouth;
- burning and pain in the eyes;
- dry cough, frequent sneezing.
With moderate and severe chlorine intoxication, the following symptoms are observed:
- severe hoarseness of the vocal cords;
- nausea and vomiting;
- decreased blood pressure and heart rate;
- headaches and dizziness;
- general weakness of the body;
- dry painful cough, turning into a wet cough with pink foamy discharge;
- convulsive syndrome;
- unproductive shallow breathing, shortness of breath;
- acute pain in the chest area, significantly aggravated by coughing;
- suffocation, short-term cessation of breathing.
The moderate and severe form of chlorine intoxication is conventionally divided into two periods: latent, lasting for four to six hours, and the period of pulmonary edema, which usually lasts three to four days, usually ending with recovery with possible serious complications due to the addition of an infectious process.
The fulminant form of chlorine poisoning is characterized by sharp spasms of the larynx, during which the glottis narrows greatly, which entails:
- cyanosis;
- respiratory arrest;
- convulsions and loss of consciousness;
- involuntary acts of urination and defecation;
- swelling of veins in the neck and face.
This condition develops within five to thirty minutes and is usually fatal.
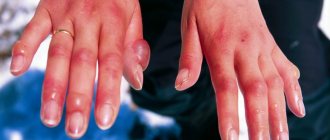
If a chlorine solution gets on the skin or mucous membranes, a skin rash, itching and burning of the skin, and the development of contact dermatitis often appear. When swallowing a chlorine-containing substance, the above symptoms are also accompanied by severe pain in the abdomen, pain and diarrhea.
Ammonia
Ammonia is another gas dangerous to humans. It has a sharp, specific smell.
Ammonia is used in industry, agriculture and medicine (in the production of ammonia).
Poisoning with significant doses of ammonia always causes death.
The symptoms of ammonia poisoning are as follows:
- lacrimation;
- Severe runny nose;
- Involuntary secretion of saliva;
- Facial redness;
- Cough of a convulsive nature;
- Suffocation;
- Pain in the sternum;
- Convulsive syndrome;
- Vomit.
Death occurs with clinical manifestations of acute heart failure.
Long-term exposure to ammonia vapors on the human body does not pass without leaving a trace. Even after successful treatment, the patient may experience neurological disorders: nervous tics, memory loss, significant deterioration in vision and hearing.
First aid for ammonia poisoning should be provided only in a special suit, gloves and shoes. The use of a gas mask is also mandatory.
Let's consider the procedure for providing first aid for ammonia poisoning:
- Protect people from exposure to toxic substances;
- Provide fresh air or oxygen inhalation;
- Rinse the nasal and oral cavities with water for up to 15 minutes;
- Drop Dicaine 0.5% into each eye;
- Ensure that there is no bright light (put the victim on dark glasses or cover the eyelids with a dark bandage);
- If the skin is damaged by chemicals, it is thoroughly washed with water and a bandage soaked in an antiseptic solution is placed on the site of the injury;
- If poison gets inside, gastric lavage is performed.
Given the high probability of death, all poisoned persons must be hospitalized.
Possible consequences and prevention of chlorine poisoning
Prevention of chlorine poisoning consists of the following measures:
- ensuring proper working conditions in accordance with sanitary and technical requirements (ventilation, airing, working equipment);
- use of personal protective equipment when working with chemicals at work;
- regular checks of chlorine concentrations in the air of the work area;
- medical examinations to identify predispositions (preclinical forms) and chronic diseases;
- in everyday life - compliance with safety requirements for the use of chlorine-containing liquids.
Considering the significant risk and widespread use of chlorine, the severity of its damage and the high possibility of death, everyone should have an algorithm of actions and a clear position - preventing poisoning is easier and more expedient than treating and dealing with its consequences.
With mild forms of chlorine intoxication, severe complications are usually absent. Recovery occurs a few days after poisoning.
Severe and moderate forms of intoxication are characterized by the development of acute and chronic complications of the following plan:
- respiratory tract diseases: laryngitis, bronchitis, pharyngitis, tracheobronchitis, tracheitis;
- toxic pneumonia and pulmonary edema;
- conjunctivitis;
- acute heart failure;
- bronchiectasis;
- paralysis of the motor and respiratory centers;
- pneumosclerosis and emphysema.
To protect yourself from such consequences and from the possibility of chlorine poisoning, in principle, you must vigilantly follow safety rules when working with hazardous substances at work, as well as at home:
- Do not work with chlorine-containing substances in poorly ventilated areas with low oxygen availability.
- Use personal protective equipment (rubber gloves, respirators, gauze masks) when working with substances containing chlorine.
- Work with pesticides and insecticides only in safety glasses and a respirator.
- Do not swallow water in a public pool.
- Keep poisons and household chemicals out of the reach of children.
Prevention of poisoning by this substance consists of following safety precautions when working with chlorine and using insulating protective equipment.
Chemical properties
Structure of the electron shell
The valence level of a chlorine atom contains 1 unpaired electron: 1S² 2S² 2p6 3S² 3p5, so a valence of 1 for a chlorine atom is very stable. Due to the presence of an unoccupied d-sublevel orbital in the chlorine atom, the chlorine atom can exhibit other valences. Scheme of formation of excited states of an atom:
| Valence | Possible oxidation states | Electronic state of the valence level | Example connections |
| I | +1, −1 | 3s2 3p5 | NaCl, NaClO |
| III | +3 | 3s2 3p4 3d1 | NaClO2 |
| V | +5 | 3s2 3p3 3d2 | NaClO3 |
| VII | +7 | 3s1 3p3 3d3 | NaClO4 |
Chlorine compounds are also known in which the chlorine atom formally exhibits valency 4 and 6, for example ClO2 and Cl2O6. However, these compounds are radicals, meaning they have one unpaired electron.
Interaction with metals
Chlorine reacts directly with almost all metals (with some only in the presence of moisture or when heated):
Cl2 + 2Na → 2NaCl 3Cl2 + 2Sb → 2SbCl3 3Cl2 + 2Fe → 2FeCl3
Interaction with non-metals
With non-metals (except carbon, nitrogen, oxygen and inert gases), it forms the corresponding chlorides.
In the light or when heated, it reacts actively (sometimes with explosion) with hydrogen according to a radical mechanism. Mixtures of chlorine with hydrogen, containing from 5.8 to 88.3% hydrogen, explode upon irradiation to form hydrogen chloride. A mixture of chlorine and hydrogen in small concentrations burns with a colorless or yellow-green flame. Maximum temperature of hydrogen-chlorine flame 2200 °C:
Cl2 + H2 → 2HCl 5Cl2 + 2P → 2PCl5 2S + Cl2 → S2Cl2
With oxygen, chlorine forms oxides in which it exhibits an oxidation state from +1 to +7: Cl2O, ClO2, Cl2O6, Cl2O7. They have a pungent odor, are thermally and photochemically unstable, and are prone to explosive decomposition.
When reacting with fluorine, not chloride is formed, but fluoride:
Cl2 + 3F2 (g) → 2ClF3
Other properties
Chlorine displaces bromine and iodine from their compounds with hydrogen and metals:
Cl2 + 2HBr → Br2 + 2HCl Cl2 + 2NaI → I2 + 2NaCl
When reacting with carbon monoxide, phosgene is formed:
Cl2 + CO → COCl2
When dissolved in water or alkalis, chlorine dismutates, forming hypochlorous (and when heated, perchloric) and hydrochloric acids, or their salts:
Cl2 + H2O → HCl + HClO 3Cl2 + 6NaOH → 5NaCl + NaClO3 + 3H2O
Chlorination of dry calcium hydroxide produces bleach:
Cl2 + Ca(OH)2 → CaCl(OCl) + H2O
The effect of chlorine on ammonia, nitrogen trichloride can be obtained:
4NH3 + 3Cl2 → NCl3 + 3NH4Cl
Oxidizing properties of chlorine
Chlorine is a very strong oxidizing agent.
Cl2 + H2S → 2HCl + S
Reactions with organic substances
With saturated compounds:
CH3-CH3 + Cl2 → C2H6-xClx + HCl
Attaches to unsaturated compounds via multiple bonds:
CH2=CH2 + Cl2 → Cl-CH2-CH2-Cl
Aromatic compounds replace a hydrogen atom with chlorine in the presence of catalysts (for example, AlCl3 or FeCl3):
C6H6 + Cl2 → C6H5Cl + HCl
How can you get poisoned by chlorine?
Most often, chlorine and chlorine-containing substances are poisoned in the following situations:
- when inhaling gas vapors;
- in case of accidental use of pesticides or liquids containing chlorine;
- in case of violation of safety regulations when working at industrial enterprises;
- when visiting a public swimming pool without proper disinfection;
- when an aqueous solution of chlorine in high concentrations comes into contact with mucous membranes or skin;
- when taking substances containing chlorine in an attempt to commit suicide.
Chlorine is especially dangerous for young children, who may accidentally ingest chlorine-containing substances. Often such poisonings lead to death.
Diagnostics
Diagnosis is carried out on the basis of examination of the victim, medical history (presence of data on leakage of chlorine vapor), objective examination (lacrimation, hoarseness, barking cough, shortness of breath, decreased blood pressure, dry or moist rales during auscultation, convulsions).
And also, if possible, laboratory examination methods - a decrease in the liquid fraction of blood, an increase in hematocrit, coagulability, pH - less than 7.3. On the ECG - partial or complete atrioventricular block, fibrillation, asystole.
Reduction of saturation indicator (determined by an external pulse oximeter) to 80%.
The entire process of treating these poisonings can be divided into three stages: first aid, inpatient treatment and rehabilitation.
How to help a victim of chlorine poisoning
What to do first in case of chlorine poisoning? Before doctors arrive, first aid should be provided to the victim.
- Eliminate the source of poison entering the body - remove or remove the patient outside the zone of action of the toxic substance. In this case, it is necessary to remember the safety of the rescuer - the use of a gauze mask or respirator.
- Provide access to clean air.
- Remove contaminated clothing and wash contact areas of skin with warm (not hot) water.
- In case of oral ingestion (swallowing) of chlorine-containing liquids, the stomach must be rinsed. It is better to rinse through a tube, or you can induce vomiting after drinking heavily.
- In case of eye damage, wash them with plenty of water or a weak solution of soda to relieve irritation.
- Rinsing the mouth and nose with soda solutions to minimize damage to the mucous membranes, using inhalations with the addition of soda to relieve cough.
In case of chlorine poisoning, first medical aid consists of ensuring airway patency (if necessary), administering Hydrocortisone or Prednisolone, and instilling anesthetic into the eyes (a solution of 0.5% Dicaine or 0.25% Novocaine).
Bleach got into my eyes, what should I do?
Bleach can get on the mucous membranes of the eyes if it is used carelessly. For example, while cleaning with this cleaning product, a person may automatically scratch their eye. In this case, bleach gets from the gloves onto the mucous membrane. This can lead to decreased vision.
To avoid serious consequences if bleach gets into your eyes, you need to take a few simple steps.:
- Remove contaminated gloves and make sure that your hands are not contaminated with chlorine solution;
- Rinse eyes with plenty of clean water;
- Place Albucid or Novocaine drops into the eyes;
- Call an ambulance or go to the ophthalmology department yourself.
In this case, you should not touch your eyes with your hands or other objects, as this may aggravate the situation.
If bleach gets into your eyes, you should consult an ophthalmologist as soon as possible. The doctor will be able to assess the extent of damage to the eye apparatus and prescribe adequate treatment.
First aid for chlorine poisoning
First of all, the victim must be urgently removed from the lesion. In case of direct contact with a chlorine-containing substance, immediately stop entering it into the body. At the same time, we should not forget about the safety of the rescuer himself. In such cases, it is imperative to use gauze bandages or respirators.
Then you need to immediately call emergency help and only then begin providing first aid to the victim.
How you can help a person with chlorine poisoning:
- Unbutton your clothes, open the window, letting in fresh air and thereby providing the victim with access to oxygen.
- Rinse your mouth and nasal passages, and thoroughly rinse your eyes with plenty of running water or a two percent solution of baking soda.
- Provide the victim with alkaline drink: milk, mineral water.
- You can use soda inhalations.
- Provide the patient with emotional and physical peace.
- If liquids containing chlorine are swallowed, it is necessary to rinse the stomach by mechanically inducing vomiting after drinking heavily.
After providing first aid, the patient must be taken to a medical facility for further treatment. The first day the patient should be under the supervision of qualified personnel, as this will help to avoid many unpleasant serious consequences and prevent the occurrence of possible serious complications.
When treated in a hospital, therapy is aimed at restoring the patient:
- supplying the victim with oxygen in the required quantity;
- stabilization of blood circulation;
- supporting the stable functioning of the patient’s vital organs: heart, lungs, stomach and others;
- normalization of metabolic processes;
- prevention of possible complications;
- symptomatic therapy.
Urgent actions
First aid for acid poisoning has several specific features:
- Gastric lavage with water with burnt magnesia dissolved in it;
- If it is impossible to carry out manipulations with gastric lavage, the patient is allowed to drink large quantities of liquid that envelops the walls of the stomach (milk, vegetable oil, rice broth);
- A heating pad with ice is placed on the abdominal area to reduce pain;
- In case of hydrocyanic acid poisoning, an antidote must be administered immediately.
If there is the slightest suspicion of a perforation of the stomach, it is prohibited to rinse it.
In case of chemical poisoning with alkalis, first aid is carried out according to the same principle.
It should be noted that the consequences of the effects of ahs on the human body are extremely negative.
Despite the fact that first aid and treatment will be correct and timely, the victim may still have the following consequences of poisoning:
- As a result of severe burns to the vocal cords, a person loses the ability to speak;
- When the esophagus is burned, scars remain on the organ, which causes pain with any meal;
- Exposure to alkalis and acids on the eyes leads to complete blindness.
All household chemicals contain compounds of alkalis and acids. Therefore, first aid for poisoning with household chemicals is provided according to the principle described above.
Signs of chlorine poisoning
The first signs of chlorine poisoning include:
- discomfort and irritation of the respiratory tract;
- increased salivation and spasm of the vocal cords;
- cough and difficulty breathing;
- sensation of cutting and burning in the eyes, lacrimation;
- nausea and bitterness in the mouth;
- headaches and possible seizures;
In case of contact with the skin or mucous membranes, significant itching and hyperemia (redness) are observed, subcutaneous hemorrhages are possible without damaging the integrity of the skin.
The severity of the pathological process and symptoms of chlorine poisoning are directly dependent on the dose of the toxic substance (chlorine) and the duration of its action.
Pathogenesis
Chlorine belongs to the group of asphyxiating and metabolic poisons. When exposed to small doses of a gaseous toxicant, the victim experiences signs of chemical irritation of the mucous membrane of the eyes and respiratory tract. At concentrations above 40-80 mg/m3, a spasm of the respiratory tract and reflex cessation of breathing develops. There is damage to the trachea, bronchi, and larynx, which creates a mechanical obstacle to the air flow. In severe cases, the patient develops symptoms of alveolar pulmonary edema.
Insufficient gas exchange provokes the occurrence of respiratory acidosis. The production of carbon dioxide exceeds the level of its removal through the lungs. The functioning of all receptor systems of the body is disrupted, and significant deviations of homeostasis from normal indicators are noted. The first to suffer is the activity of the cardiovascular and central nervous systems. Irreversible changes in the brain associated with prolonged oxygen deprivation often occur.
Causes
In most cases, chlorine poisoning occurs when this component leaks during accidents at industrial enterprises. Damage to large containers is accompanied by massive toxic exposure to employees and leads to chemical contamination of nearby water bodies, populated areas, and natural resources. The area of pollution depends on the volume of emissions, ambient temperature, and wind speed.
The accumulation of chlorine sometimes occurs during scientific experiments, which are accompanied by the release of this gas. In this case, intoxication can be provoked by the lack of respiratory protection and insulating clothing, poor ventilation in the room, and refusal to use equipment that signals the accumulation of toxic substances in the room.
Sometimes visiting a swimming pool can also cause chlorine toxicity. This is possible when using outdated water disinfection technology. When using high doses of hypochlorite as an antiseptic, there is a risk of poisoning people, since the largest amount of xenobiotic accumulates in the upper layers of water.
The use of chlorine-containing detergents in hospitals where chlorine tablets are used is often accompanied by poisoning of orderlies. The main cause of damage is exceeding the recommended dosages and non-compliance with the rules for working with antiseptic compounds.
Chlorine poisoning is possible in the following cases:
- exceeding the maximum permissible concentrations of chlorine for disinfecting water in the pipeline (strong smell of chlorine);
- the presence of chlorine in large quantities in the pool water and frequent swimming in it;
- bleaching and washing in a closed, unventilated area;
- accidents at the enterprise;
- the use of chlorine as a weapon of mass destruction.
Chlorine enters the human body through the mucous membranes of the respiratory and digestive systems and skin.
Isotopic composition
There are 2 stable isotopes of chlorine found in nature: with a mass number of 35 and 37. The proportions of their content are respectively 75.78% and 24.22%.
| Isotope | Relative mass, a.m.u. | Half life | Type of decay | Nuclear spin |
| 35Cl | 34.968852721 | Stable | — | 3/2 |
| 36Cl | 35.9683069 | 301000 years | β-decay in 36Ar | 0 |
| 37Cl | 36.96590262 | Stable | — | 3/2 |
| 38Cl | 37.9680106 | 37.2 minutes | β-decay in 38Ar | 2 |
| 39Cl | 38.968009 | 55.6 minutes | β-decay in 39Ar | 3/2 |
| 40Cl | 39.97042 | 1.38 minutes | β-decay in 40Ar | 2 |
| 41Cl | 40.9707 | 34 s | β decay in 41Ar | |
| 42Cl | 41.9732 | 46.8 s | β-decay in 42Ar | |
| 43Cl | 42.9742 | 3.3 s | β-decay in 43Ar |
Receipt history.
Also on the topic:
PERIODIC SYSTEM OF ELEMENTS
Chlorine was probably obtained by alchemists, but its discovery and first research are inextricably linked with the name of the famous Swedish chemist Carl Wilhelm Scheele. Scheele discovered five chemical elements - barium and manganese (together with Johan Hahn), molybdenum, tungsten, chlorine, and independently of other chemists (albeit later) - three more: oxygen, hydrogen and nitrogen. This achievement could not be repeated by any chemist subsequently. At the same time, Scheele, already elected as a member of the Royal Swedish Academy of Sciences, was a simple pharmacist in Köping, although he could have taken a more honorable and prestigious position. Frederick II the Great himself, the Prussian king, offered him the post of professor of chemistry at the University of Berlin. Refusing such tempting offers, Scheele said: “I cannot eat more than I need, and what I earn here in Köping is enough for me to eat.”
Numerous chlorine compounds were known, of course, long before Scheele. This element is part of many salts, including the most famous - table salt. In 1774, Scheele isolated chlorine in free form by heating the black mineral pyrolusite with concentrated hydrochloric acid: MnO2 + 4HCl ® Cl2 + MnCl2 + 2H2O.
Also on topic:
GAS
At first, chemists considered chlorine not as an element, but as a chemical compound of the unknown element muria (from the Latin muria - brine) with oxygen. It was believed that hydrochloric acid (it was called muric acid) contains chemically bound oxygen. This was “testified”, in particular, by the following fact: when a chlorine solution stood in the light, oxygen was released from it, and hydrochloric acid remained in the solution. However, numerous attempts to “tear” oxygen from chlorine led nowhere. Thus, no one has been able to obtain carbon dioxide by heating chlorine with coal (which, at high temperatures, “takes away” oxygen from many compounds containing it). As a result of similar experiments carried out by Humphry Davy, Joseph Louis Gay-Lussac and Louis Jacques Thenard, it became clear that chlorine does not contain oxygen and is a simple substance. The experiments of Gay-Lussac, who analyzed the quantitative ratio of gases in the reaction of chlorine with hydrogen, led to the same conclusion.
In 1811, Davy proposed the name “chlorin” for the new element - from the Greek. "chloros" - yellow-green. This is exactly the color of chlorine. The same root is in the word “chlorophyll” (from the Greek “chloros” and “phyllon” - leaf). A year later, Gay-Lussac “shortened” the name to “chlorine.” But still the British (and Americans) call this element “chlorine”, while the French call it chlore. The Germans, the “legislators” of chemistry throughout almost the entire 19th century, also adopted the abbreviated name. (in German chlorine is Chlor). In 1811, the German physicist Johann Schweiger proposed the name “halogen” for chlorine (from the Greek “hals” - salt, and “gennao” - give birth). Subsequently, this term was assigned not only to chlorine, but also to all its analogues in the seventh group - fluorine, bromine, iodine, astatine.
In 1826, the Swedish chemist Jons Jakob Berzelius, having refined the data he had previously obtained, determined the atomic mass of chlorine to be 35.41, which differs from the modern one by only 0.1%! This is an amazing result considering the quality of the equipment with which the famous chemist worked. The main tool for determining atomic masses is a balance. Once upon a time, each copy of precise analytical balances was made by hand by a master, and good scales were very expensive. Therefore, only a few very rich chemists could boast of such scales. Berzelius himself in his youth had a poorly equipped laboratory with rather crude scales, so in order to obtain reliable results he was forced to repeat the same analysis 20–30 times! Within 10 years, Berzelius published the results of the analysis of 2000 compounds formed by 43 elements, and the work he spent on this colossal work surpasses all imagination. Almost a century later, another famous chemist, one of the first Nobel Prize laureates in chemistry, Wilhelm Ostwald, seeing the equipment with which Berzelius worked in the museum, said: “It became completely clear to me how little depends on the device and how much depends on the person in front of it.” is sitting".