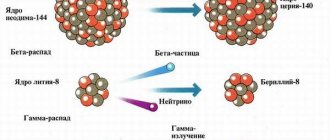
Due to the fact that the human body does not feel the effects of radiation, its existence was unknown for a long time. The accidental discovery of Becquerel at the end of the 19th century, who discovered photographic film exposed from a piece of uranium, marked the beginning of its study. Maria Sklodowska and Pierre Curie began researching the ability of certain substances to emit X-rays. At the same time, the term radioactive substances appeared.
Marie Sklodowska-Curie died in 1934, becoming the first victim of radiation sickness. The scientist carried a test tube with radium with her all the time, kept it on her desk, and was exposed to constant radiation. Maria's personal books and work journals are even now stored in special boxes covered with sheets of lead because they emit radiation.
At the same time, Roentgen discovered cathode rays emitted by a cathode ray tube. Cathode radiation is radioactive, but is of artificial origin. At the beginning of the 20th century, a device for measuring radioactive gamma radiation was created - the Geiger counter.
Thus began the era of studying radiation and the effects of radiation on the human body.
Curie, Roentgen and Geiger
What is radiation?
Radiation refers to a form of energy. It arises as a result of various reactions and transformations occurring in atoms. The main distinctive features of radiation energy waves are:
- the ability to penetrate any body, be it a person, plants or solid concrete;
- the ability to change molecules as they pass through the body or destroy DNA when interacting with living organisms. Such changes are irreversible; it is this feature of radiation that affects the appearance of numerous mutations.
The effect of radiation on the human body has not yet been fully studied. This is especially true for genetic changes that occur in the body and can be inherited.
Important! Radiation should not be confused with a chemical reaction. When two substances interact, no radioactive radiation is released!
Radiation and its features
The particles that create radiation fall from the nucleus of an atom of elements (uranium and others). Radioactive decay occurs in the core itself. One element can have several variants - isotopes, and some of them will be radioactive, while others will be stable.
Each of the radioactive isotopes has its own life period, ending with the decay of the nucleus. The time required for half of the isotope nuclei to decay is called the half-life. It can last from a fraction of a second to millions of years.
In nature, the formation of radioactive isotopes occurs naturally, but they can also be created artificially. This happens during the construction of nuclear power plants and nuclear tests.
Types of radioactive radiation
Radioactive radiation consists of several types of energy waves. These include:
- alpha particles are quite dangerous for the human body, but have such low penetrating ability that they cannot even penetrate human skin. Such radiation affects a person only when ingested with food or through open wound surfaces;
- beta particles are lighter than alpha particles. They are capable of penetrating a couple of centimeters into the human body, but they pose less danger than the previous ones. Special shields and clothing were created to protect against beta radiation;
- Gamma particles are a type of radioactive energy that can penetrate any surface. When interacting with living cells, gamma rays cause irreversible changes. Gamma particles are the most dangerous; to neutralize them, special concrete sarcophagi with a high lead content are erected, but some gamma rays are able to overcome these barriers;
- neutron radiation - has a similar nature to gamma rays and is just as dangerous. Neutron radiation is produced during nuclear reactions or special research, as well as during nuclear explosions. The negative effects of radiation on the human body can be partially prevented by using protection made of concrete, lead and iron.
First aid for radiation exposure
People exposed to dangerous levels of radiation for humans must receive first aid. All clothing should be removed and disposed of immediately. You need to take a shower with detergents as soon as possible. In the future, the removal of harmful substances is carried out with the help of medical measures and drugs:
- Graphene is a special carbon form that activates the removal of nuclides.
- Activated carbon, eliminating hazardous effects.
- Polyphepan, which helps the body fight the effects of radiation,
- Potassium orotate – to prevent cesium concentrations and protect the thyroid gland.
- Dimethyl sulfide with antioxidant action to protect DNA and cells.
Dietary supplements bring certain benefits. They contain iodine to eliminate the effects of isotopes that accumulate in the thyroid gland, clays with zeolites, which bind radiation waste and remove it from the body. Calcium supplements help eliminate strontium.
Radiation Exposure Limits
Radiation affects all systems of the human body, but some organs are more resistant to its effects, while others suffer almost immediately. As a result of numerous studies, scientists were able to create a scale that allows them to assess the total damage caused by radioactive radiation for each individual person. The unit of measurement was the sievert (Sv), which is the ratio of joules to kilograms. According to the scale, the following radiation doses are allocated:
- 100 Sv is a lethal dose, the effects of radiation appear within a few minutes. Irreversible damage occurs to all systems in the body, as well as to the skin. A person’s death occurs within a few hours; in rare cases, the victim can live for a couple of days;
- 50-10 Sv - the victim can live from a couple of weeks to a couple of months. Death occurs as a result of numerous internal bleeding caused by radiation sickness;
- 5-4 Sv – with timely assistance and qualified therapy, a favorable outcome and recovery of a person occurs in approximately 50% of cases. Despite this, damage to the DNA and cellular structure remains unchanged, as a result of which such a person may have children with genetic abnormalities, or develop oncology several years after irradiation;
- 1 Sv – a mild form of radiation sickness develops. In this case, victims experience symptoms such as disturbances in the gastrointestinal tract, decreased libido, poor appetite, sleep disturbances and psycho-emotional state. In most cases, the disease goes away completely, but there remains a high risk of having children with abnormal abnormalities or developing tumors and blood diseases;
- 0.75-0.5 Sv - with this dose of radiation, the effects of radiation cause short-term disturbances in the human body, which are quickly neutralized. Examples include changes in blood clotting or the formation of hematomas;
- 0.05 Sv – level of radiation exposure for medical devices;
- 0.0005-0.0003 Sv – has no effect on humans, is considered the normal level of the received radiation.
Preventive measures
Regular monitoring of background radiation helps to avoid such exposure. This applies to industrial and residential premises, water, and food. During measurements, the intensity of radiation and the degree of danger of the source are taken into account, and the time that is permissible to spend near it without unpleasant consequences is determined.
The unit of measurement for the radiation received is the sievert. The value shows the amount of energy absorbed by a kilogram of biological tissue over an hour. The maximum permissible norm is considered to be 0.5 microsieverts per hour; the normal value should not be higher than 0.2 microsieverts per hour. Higher levels are a dangerous dose of radiation for humans. A reading of 5-6 sieverts is lethal .
Radioactive people who have been exposed cannot be a source of radiation. It is safe to communicate with them; radiation sickness is not transmitted this way.
Radiation sickness: how does radiation affect the body?
Depending on the duration of exposure and dosage of radiation, we can talk about one or another degree of radiation sickness. Usually the disease has pronounced symptoms, but in some cases it is completely asymptomatic. The development of radiation sickness is possible in the following cases:
- with external irradiation for a short time, but with a high dose of radiation (acute type);
- with regular exposure to small doses, for example, when working in certain types of production (chronic type);
- when a radiation source enters the body.
Acute radiation injury
Symptoms of acute radiation injury depend on the single dose of radiation and the duration of the effect of radiation on the body. The most characteristic signs include:
- general deterioration of health, weakness, drowsiness, chronic fatigue;
- disturbances in the gastrointestinal tract in the form of diarrhea and abdominal pain;
- changes in blood composition;
- baldness (starts within a week);
- confusion;
- anemia;
- bleeding, both internal and external, for example, nose or gum.
In addition to the above, a person experiences extensive damage to the skin: the tissues acquire a bluish or purple tint, wounds and necrotic ulcers form. Pneumonia, encephalitis, hepatitis, etc. are diagnosed as complications. If a person manages to cope with the consequences of radiation and survive, then in the future he is at risk for developing cancer. It is impossible to completely neutralize the effects of radiation on humans in large doses.
Chronic form of the disease
Since in the chronic form of the disease the body is irradiated in small doses over a long period of time, the symptoms of radiation sickness do not appear immediately. As a result of the cumulative effect, damage to internal organs lasts for weeks, months and even years. Depending on the severity, radiation affects the body as follows:
- gradual decrease in performance, the appearance of chronic fatigue, drowsiness;
- unreasonable mood swings, depression;
- problems with the gastrointestinal tract, development of chronic gastritis, ulcers;
- the appearance of bruises on the body and vascular network;
- problems with the cardiovascular system;
- frequent nosebleeds and bleeding gums;
- headache;
- loss of teeth and hair;
- formation of ulcers and necrotic surfaces on the skin.
If the effects of radiation are not eliminated, the person will eventually die from radiation sickness. It is worth noting that even during the treatment of malignant diseases, radiation has a negative effect on the body. In this regard, during treatment the patient is isolated from other patients.
Signs of exposure
Signs of radiation exposure are:
- vomit,
- disorientation,
- the appearance of ulcers on the body that cannot be treated,
- bleeding from the mouth, nose, rectum,
- diarrhea with blood,
- radiation burns on the skin,
- hair loss,
- feeling weak and tired,
- fainting, headache,
- sores on the lips and mouth,
- tremors, seizures,
- fever.
In people who have received a dose of radiation, blood pressure drops, heart function and vascular tone are disrupted. Hepatitis and cirrhosis of the liver may develop, and the functioning of the biliary system may malfunction. The level of leukocytes in the blood sharply decreases.
All this is far from a complete list of how radioactive substances are dangerous to humans. The changes that occur affect the entire body and have a negative impact on all its systems.
Radiation sickness prognosis
The effect of large doses of radiation on the human body is deadly. Doctors identify a critical period, which is 12-14 weeks. If the patient survives this time limit, then he has every chance of recovery. However, despite this, he may develop malignant tumors in the future. When the body is exposed to a lethal dose of radiation, there is no treatment. The only way to prevent the negative effects of radiation on the body is to follow the rules of prevention.
Radiation: risks, safety, protection
The word “radiation” evokes fear in most of today’s readers. Radiation is associated with death. An invisible, unheard, imperceptible killer, slowly killing - maybe you too, reader? Should you be afraid? The answer is in this article. KDPV - from the book “Physicists Are Joking.”
The first "bells"
The understanding that ionizing radiation has a certain physiological effect on the body was already among its first researchers.
The fact that Konrad Roentgen's X-rays cause burns was discovered the hard way by his assistant W. Grubbe almost immediately after their discovery. The discoverer of uranium rays, Henri Becquerel, also felt their effects on himself when he put an ampoule with radium salt in his pocket to show it to his students: the skin around the ampoule became red and painful, and then a long-healing ulcer formed. Many patients who were exposed to x-rays and the doctors who treated them suffered burns and ulcers from exposure to x-rays, and an employee of Thomas Edison’s laboratory, who worked for a long time at a public demonstration of x-rays, lost his legs from radiation burns, and subsequently died early from skin cancer. By 1907, at least seven deaths from ionizing radiation were already known, and the total number of radiologists who died from radiation during the first decades of its use was in the hundreds. Despite this, the public greeted the new phenomenon with enthusiasm. The discovery of the therapeutic effect of X-rays and radium rays on such a terrible and incurable disease as cancer and the discovery of the stimulating effect of weak radiation on life processes led to the fact that ordinary people saw radium as a panacea. Radioactive mineral water, radioactive toothpastes and cosmetics, and devices for saturating water with radon containing radium went on sale. Fortunately, in most cases they were only radioactive in advertising. However, the drug "Raditor", which was present on the shelves of pharmacies for ten years from 1918 to 1928 and actually contains a microgram in each bottle
radium-226.
It was recommended to take a bottle a day.
For reference: at a distance of 1 cm
, a microgram of radium creates a dose rate of
8.4 mr/h
of gamma radiation alone.
The permissible intake of radium-226 per year
(NRB-99) is 35
nanograms
.
Raditor was claimed to be a cure for all diseases, including impotence, rheumatism and schizophrenia.
It is unknown how many lives it claimed - we only know about the death of Eben Byers, an American millionaire and industrialist, from oral cancer, which developed after taking about one and a half thousand vials over several years. Perhaps the most famous victim of radiation of that time was one of the pioneers of the radioactive field, Marie Skłodowska-Curie, who died of radiation-induced leukemia in 1934. Both Henri Becquerel and Irene Joliot-Curie probably died early from the doses of radiation they received. Now it is no longer possible to find the names of all those who died and became seriously ill while working in those years with enormous activity without any protection or precautions, but apparently there were many of them.
From then on, only a little more than ten years passed until the moment when deadly radiation showed itself on all sides in Hiroshima and Nagasaki. Then there was a lot of things: a girl folding cranes, and test explosions through the epicenters of which companies of soldiers were driven, and Mayak, and Chernobyl...
Effect of radiation on matter and living tissue
It all starts with the act of ionization - one of the electrons of the atom is given an energy that exceeds the energy of its connection with the atom and it flies away, leaving the atom with a positive charge.
But the energy of a gamma radiation quantum, alpha or beta particle is too great for it to end there. Ionization energy is measured in units, a maximum of the first ten electron volts, and the energy of a particle or quantum can be megaelectron volts. Therefore, as a result of a single act of interaction, thousands and tens of thousands of atoms are ionized. The electrons ejected from them also acquire energy sufficient to ionize other atoms, and everything continues until, in the end, the energy of the next electrons is lower than the ionization energy. What is the result? The transformation of a neutral atom into an ion, firstly, weakens or destroys the previous chemical bonds that this atom formed, and secondly, it makes this atom an extremely active reaction center that instantly forms new chemical bonds.
When it comes to a crystal, this leads to the formation of point defects in the crystal lattice - radiation defects, which gradually, as the dose accumulates, change the properties of the material. The metal becomes more brittle, the conductivity of silicon increases and the mobility of charges decreases, optically transparent materials become less transparent, become colored, dielectrics begin to “flow” - materials “get tired” of the accumulated dose and collapse, stop working as they should, and those made from them devices fail. In the limit, the crystal turns into an amorphous substance. Many uranium and thorium minerals are found in such - metamict
state: during the time that has passed since their formation, the radiation emitted by them completely destroys the crystal lattice, while the shape of the crystals remains the same.
But living matter is no better in this sense. If in a protein molecule one of the amino acids turns into who knows what, even if the protein chain does not break, such a protein molecule will no longer perform its function. If one of the lipid molecules in the membrane, having turned into an active ion, reacts with a neighboring molecule and the resulting “Frankenstein” ceases to be a structural element of the membrane - a hole will remain in it. Excess molecules that no longer perform their functions remain in the cell and interfere with its work, poisoning it. And the worst thing is if the most important molecule in the cell gets damaged - the DNA molecule that carries genetic information. This will lead to distortion of the latter, the appearance of mutations.
Ionization followed by neutralization of the resulting ionized fragments leads to the formation of free radicals, which interact with neighboring molecules and destroy them, transferring to them an unpaired electron and, along with it, reactivity. And so on - until two radicals meet... Therefore, in order to damage a molecule, it does not have to come directly under the influence of a high-energy particle - radicals continue its destructive work. Their existence time is short - from nano- to microseconds, but it is much longer than the time of the act of interaction itself.
Having received a radiation “hit”, the cell first tries to recover. Mechanisms for eliminating molecular “garbage” are activated, dead molecules are re-synthesized, leaky membranes are patched, and repair mechanisms try to “sew together” broken chromosomes. If everything is really bad, the cell launches a self-destruction program - apoptosis.
The worst situation is for those cells that are actively dividing. Everything about them is vulnerable and they have difficulty recovering. Therefore, tissues in which constant cell division and growth occur—bone marrow tissue, gonad tissue, embryonic tissue—are the most radiosensitive and are the first to suffer when irradiated.
Radiation sickness
Massive cell death and suspension of the functioning of survivors after acute irradiation adversely affects the functioning of the affected organs, and therefore the body as a whole.
Toxic cell breakdown products, free cellular enzymes, cytokines and other signaling molecules, radiolysis products are released into the bloodstream, which aggravates the severity of the lesion. Acute radiation sickness develops. Its beginning looks like poisoning with something unknown, and it is really poisoning with everything that immediately after irradiation entered the bloodstream as a result of massive cell damage. Vomiting begins, pressure drops, temperature rises - this is the so-called primary reaction. It goes away and the person gets better. It seems that everything is already behind us - but in fact, the main problems have not yet shown themselves. And they are already serious: the bone marrow is partially or completely lost. At a dose of 100 rem, 20% of bone marrow cells are nonviable. At a dose of 500-600 rem, the bone marrow is completely dead. As long as the existing blood cells are working, everything is fine. But their service life is several days, and they need to be replaced. But replacement won’t come from nowhere. The body becomes defenseless against infections, the blood loses coagulability, and its ability to transport oxygen and carbon dioxide decreases.
The first signs of radiation sickness appear when the absorbed dose of gamma radiation is about 1 Gy. Lower doses do not cause clinical manifestations, although certain pathological changes in blood and bone marrow tests are detected at doses of tenths of a gray. At doses up to 5-6 Gy, as long as there are still viable dividing progenitor cells in the bone marrow, there is a chance of recovery. At doses less than 2 Gy, this chance is absolute and recovery is complete, and up to 4 Gy, the probability of dying is small, but the consequences in half of the cases will remain forever. Above 6 Gy, there is some possibility of “pulling out” a person using a bone marrow transplant from a donor, but when the dose exceeds 10 Gy, not only he, but also the precursor cells of the intestinal epithelium die. This is already absolutely deadly. Moreover, after the initial reaction to radiation has passed, the so-called walking corpse phase often begins: the person feels quite tolerable, nothing hurts, his strength has returned: the body functions on old blood cells, on the old intestinal epithelium. When they end, and this will happen very soon, in a few days or even hours, the imaginary “health” will end (with bloody diarrhea and then painful death).
At very high doses of hundreds of grays, the most radioresistant cells die. Those that do not divide are nervous and muscular. The victim of radiation immediately begins to experience symptoms of brain damage: convulsions, psychomotor agitation, followed by depression of consciousness up to coma, and within a short time (from several hours to several days) - death. In popular literature they often talk about “death under the ray,” the instantaneous death of the entire organism right at the moment of irradiation, but this is rather a theoretical assumption that doctors have not yet encountered.
It must be said that 1000 Gy is a very large dose in terms of its effect on living matter, but even such a dose is a rather small amount if you look at the absorbed energy, which can heat living tissue by only 0.3 ° C.
Stochastic effects or low dose diseases
Radiation sickness is a disease that has a pronounced threshold for the onset of its manifestation, and its severity is proportional to the radiation dose.
This is the so-called deterministic effect of radiation. However, if the dose is insufficient to cause radiation sickness, this does not mean that the radiation exposure has passed without a trace. But the manifestation of this “trace” left by irradiation becomes fundamentally different. The primary cause of death of bone marrow cells during irradiation is usually gross damage to their genetic apparatus - so-called chromosomal aberrations. Pieces break off from the chromosomes, which can be attached to other chromosomes, ring-shaped chromosomes are formed, etc. But such damage does not always lead to immediate cell death. As a result of chromosomal rearrangement, and sometimes even as a result of a point mutation - the replacement of only one or several nucleotides in DNA - one or more mechanisms regulating cell division and differentiation are disrupted. Cell division becomes uncontrollable and it gives rise to a population of tumor cells
, which under certain circumstances develops into a malignant tumor. The most easily and quickly caused by irradiation are tumors of the hematopoietic system - leukemia, less often these are oncological diseases of other localizations. In addition, from radiation exposure to the development of leukemia usually takes a short time - 1-2 years, or even less, and the development of cancer until the appearance of a detectable tumor or clinical manifestations often takes more than ten years.
But the very occurrence of mutation is a consequence of a single act of interaction of the cell nucleus with a gamma radiation quantum or high-energy particle. An unpleasant consequence follows from this: unpleasant consequences that threaten the death of the entire organism can be caused by the entry of a single
particles.
Fortunately, with a very low probability. The second consequence is the independence of the severity of the lesion from the dose and the fact that only the probability of
its development depends on it. This probability is estimated at approximately 5% per gray of absorbed dose and is presumably proportional to it.
In addition to cancer, there are also germ cell mutations. Everything is the same here: the severity of the manifestation of the mutation does not depend on the dose (it depends on which gene and how it was damaged, but the nuclear particle does not choose which part of the DNA molecule it hits), only the probability of the occurrence of mutations depends on it.
Such effects, unlike deterministic effects, are called stochastic
, emphasizing their random, probabilistic nature
Is there a threshold or no threshold?
In radiology, from the very beginning of its existence, there has been a debate: is there a threshold for stochastic effects, or is it even the natural background that causes oncology?
On the one hand, repair mechanisms are constantly operating in the cell, which manage to promptly eliminate all or almost all damage, and catastrophic damage with chromosomal aberrations is extremely rare at natural levels of radiation. And the vast majority of studies concerning the frequency of manifestation of stochastic effects were done at acute doses of at least a few tenths of a gray, when there is a high probability of multiple damage to the same cell before its self-repair is completed. Therefore, it is likely that in the low-dose region the frequency of stochastic effects per gray of absorbed dose may be significantly lower than in the high-dose region. But it is very difficult to verify whether this is so. The reason for this is that a person suffers from cancer and has nothing to do with radiation. And he gets sick often: 20% of the world's population faces cancer. Against this background, detecting a small addition to the dose on the order of the natural background (2.4 mSv/year over 70 years of life is 168 mSv, which contributes less than a percent to the overall incidence of oncology) is extremely difficult to detect, simply because of the statistical scatter: this required to recruit in each of the groups (experimental and control) no less than a million completely healthy experimental subjects living in exactly the same conditions. In any case, directly
method - by studying the frequency of oncology in groups living under different natural radiation backgrounds (and in different parts of the Earth it can range from 3.5 to several hundred microR/h) it was not possible to identify any clear correlation of one with the other.
Another still unsolved problem is the question of whether what in photography is called the law of reciprocity works here? That is, is there a difference between the dose received in a minute, in a year or in a lifetime? At high doses, when we can talk about radiation sickness, there is undoubtedly a difference. With short-term exposure, the dose that causes radiation sickness is much less than the dose that causes chronic radiation sickness with long-term exposure.
Until these issues are resolved, safety decisions are guided by the assumption that the frequency of stochastic effects is proportional to the dose down to zero and there is no difference between acute and chronic doses. This is the so-called no-threshold concept, according to which there is a risk from any dose and we set exposure limits based on the acceptable risk
.
Hormesis or accelerated aging?
In the concept described above, there is no room for deterministic dose-dependent effects at low doses.
Nevertheless, hypotheses about the existence of such have been put forward. Moreover, both about harmful effects and about beneficial ones. Even the first experimenters in the field of radiobiology noticed: radiation stimulates plant growth, accelerates seed germination, and under conditions of a sharply lower radiation background compared to natural conditions, the division of paramecia ciliates is greatly slowed down. This phenomenon was called radiation hormesis and the assumption arose that small doses of radiation may not have a detrimental effect on higher animals and humans, but on the contrary, have a beneficial effect. Some experiments confirm this - an increased life expectancy of irradiated rodents compared to controls and increased immunity were noted. Experiments on humans are contradictory: the results of some show the presence of hormesis, while others deny it.
The opposite hypothesis is that small doses, only slightly higher than the natural background, shorten life expectancy, reduce immunity, cause cardiovascular and even neurological diseases, cause a slowdown in the development of children and a deterioration in their health. This hypothesis has its supporters; a number of articles have been published that seem to confirm it - but always on very small samples, in which statistics can play a very bad joke. In large samples, again, there is no correlation between the natural radiation background in the area and life expectancy.
This is where we will finish discussing the effects of radiation on the body and will deal with protection and safety.
About the permissible level of radiation
Opinions of instructions for household dosimeters, notes in newspapers and messages on TV and other “reliable” sources vary: figures of 30, 50, 60 microR/h were popular.
I have not found a single regulatory document that would indicate such figures. Moreover, the dose rate itself does not matter - what matters is the dose collected by a person over long periods of time - years and decades. In any case, the radiation situation is relatively calm for now. That is, it’s not like if the dosimeter shows, for example, 0.15 μSv/h, you can walk here calmly, but suddenly it shows 1.2 μSv/h and a scary red “Danger” sign — you need to quickly get away. In fact, 1.2 μSv/h is, of course, not very good numbers, but only in the case of a long stay: months, years.
In our country, the document establishing permissible exposure standards is the Radiation Safety Standards or NRB and the Basic Sanitary Rules for Radiation Safety - OSPRB. The current valid versions of these documents are SanPin 2.6.1.2523-09 NRB-99/2009 and SP 2.6.1.2612-10 OSPORB-99/2010. The UXO considers two groups: “civilians”, the population who do not work with radiation sources, and those whose work with radiation is the subject of their professional activity. The population per year (on average over five years) is allowed to gain from man-made sources of radiation
only 1 mSv. In terms of dose rate, if you count, it’s only 0.11 μSv/h, which does not include the natural background. And the latter can be anything at all. The NRB washes its hands of this, only proposing to “limit exposure from certain natural sources” (primarily, these are restrictions on the concentration of radon in the air and the specific activity of natural radionuclides in materials used in construction). So, if the natural background is approximately taken as 0.1 μSv/h, then the permissible level of radiation that acts constantly and continuously can be considered 0.21 μSv/h.
Knowing that every sievert is a 5% chance of getting cancer, we find that 1 mSv/year from man-made sources, allowed under the National Regulations, is an additional 0.35% risk of cancer over a lifetime (about 70 mSv).
On the one hand, this approach is understandable in the sense that the natural background radiation, as well as internal radiation associated with potassium-40, is a given with which nothing can be done, and it is necessary to minimize exactly that part of the dose that can be influenced.
But on the other hand, there is a certain deceit in this approach. However, the OSPRB pays somewhat more attention to the protection of the population from natural sources: there, a value of 5 mSv/year is accepted as an acceptable level of exposure from the latter, and at a level above 10 mSv/year, priority measures are required to reduce it. 5 mSv/year is 0.55 μSv/h, but we must not forget that this includes internal exposure. If we assume that it will account for approximately half of the dose, the dosimeter will show 0.23 μSv/h. That is, if where you live, the dosimeter readings exceed approximately 0.2-0.25 µSv/h (or 20-25 µR/h) - this is a reason to think about changing your place of residence, but if suddenly while walking you wander into a place where the dosimeter showed even ten or twenty times higher values - you should not panic and rush home to drink glasses of vodka to “remove radiation”. What's worth doing is to check if there is any radioactive dirt left on your soles.
All these standards do not apply to personnel working with sources of ionizing radiation - their permissible exposure standards are much higher - up to 20 mSv/year on average for 5 years, but not more than 50 mSv/year, and for the entire career - no more than 1 Sv .
About time, space and lead bricks
What to do if the radiation level is too high?
Then you need protection. And the simplest and cheapest protection is called “protection by time and distance” - stay away from the source and minimize the time of contact with it. The role of time, I think, requires no explanation. With distance it becomes more interesting. If the size of the source is small compared to the distance to it, the intensity of radiation from it obeys the inverse square law. Let's take, for example, an ampoule containing a milligram of radium. As we know, at a distance of a centimeter from it, the exposure dose rate is 8.4 R/h. By increasing this distance by 100 times, that is, up to a meter, we will reduce the radiation level by 10 thousand times, to 840 microR/h. But if we, having violated all the safety rules, take this ampoule in our hands, we will reduce the distance to the thickness of the ampoule wall, for example, to 0.5 mm. And our fingers will find themselves in the radiation field with a dose rate 400 times greater - 3360 R/h! This really is “something you shouldn’t touch with your hands”! For comparison, if you take the same ampoule with forceps with handles 30 cm long, this will reduce the radiation level by one-fold. By the way, this degree of protection is equivalent to a layer of lead more than two centimeters thick!
Unfortunately, when the source is not very similar to a point, the inverse square law ceases to apply. And radiation levels do not always allow you to limit yourself to protection with its help, and then you have to use additional protection.
Alpha and beta radiation have virtually no penetrating ability and protection against them is not a problem. The first is absorbed in a few centimeters of air, and the range of alpha particles in solid or liquid media is measured in tens or even units of microns. Beta particles usually have a longer range, but a plate of aluminum, glass or plastic with a thickness, depending on the energy, from fractions of a millimeter to a centimeter, is also impenetrable to them. It is much more difficult to protect against gamma radiation and neutrons.
Gamma radiation is absorbed primarily by electrons. The more there are on its path, that is, the higher the atomic number of the substance, the stronger the absorption will be. In the low-energy region, where the main mechanism of absorption is the photoelectric effect, it is proportional to the atomic number to the fifth (!) power; with increasing energy, the proportion of Compton absorption gradually increases, which depends linearly on the atomic number. That is why, to protect against radiation, they try to take substances with the highest possible atomic number. Lead is the most well-known material for radiation protection, but concrete and even water are also used, due to the fact that their layer can be much thicker than the layer of lead, which, although not the most expensive metal, is still expensive and harmful. And vice versa - to protect against small but evil sources used in flaw detectors, sterilization units, radiation therapy devices, RTGs - depleted uranium is often used. It is, of course, also radioactive, but its radiation danger is not comparable with the radiation of its contents - a tiny deadly ampoule with iridium-192, cesium-137 or cobalt-60. Sometimes tungsten is used - it absorbs gamma radiation less than lead, but its almost twice the density eliminates this difference.
But for neutrons the opposite is true: lead is almost transparent to them, but they are well retained by substances consisting of light atoms, especially those that contain a lot of hydrogen. A neutron, colliding with a proton, remains in place, and the proton flies further. But the latter will not fly far - having a charge, it transfers its kinetic energy to the electrons and nuclei of the atoms surrounding it. The penetrating power of protons is not much greater than that of alpha particles. True, this is not enough to protect against neutrons: once they stop, they do not cease to be in general and to be harmful in particular. But such low-energy, so-called thermal neutrons acquire the property of being reflected well from light materials - beryllium, aluminum, etc. Another important element in protecting against neutrons is boron.
Its nucleus with a mass number of 10 (which is approximately 20% of all boron atoms) greedily captures a neutron, after which the resulting nucleus immediately decays into an alpha particle and stable lithium-7. True, as a result, gamma radiation with an energy of 0.48 MeV is also produced, from which one also has to protect oneself. Therefore, modern composite materials for neutron protection include plastic, which includes boron, and a filler - lead oxide. It also absorbs hard (2.18 MeV) gamma radiation from the rather rare reactions of inelastic collision of a neutron with a proton with the formation of a deuterium nucleus. To conclude this section, I will provide a useful link to a calculator for calculating the dose rate at a given distance from the source with and without protection.
Closed sources, open sources
A source of radioactive radiation located in a hermetically sealed ampoule or otherwise reliably isolated from the release of the active substance to the outside is called a closed source.
It (at least until it is destroyed - crushed, sawn or melted, as parts happen with sources that fall into scrap metal) is a source of only external radiation. The situation is different with open radiation sources. A solution of a radioactive substance in a beaker or flask, radioactive ore, radioactive fallout, aerosols, wastewater in the environment - these are all radioactive sources. They differ from closed ones in that it is possible for radioactive substances to enter the body. In this case we have the extreme case, the opposite of “protection by time and distance”: the distance is zero and each act of decay causes damage, the time is long or even tends to infinity.
In this sense, we consider such a concept as radiotoxicity
one or another radionuclide. Taking into account the “pharmacokinetics” and “pharmacodynamics” of a radioactive substance introduced into the body and its loss due to excretion and decay, as well as the energy released during each act of decay, it is possible to determine what dose this or that activity of a given nuclide will give to various organs throughout life, and Based on this, assess the risk of stochastic and deterministic effects depending on the amount of incoming nuclide.
Dose coefficient
radionuclide is the amount of additional internal radiation dose that a person will receive per unit of activity of this radionuclide. Based on this value, it is possible to calculate the annual limit for the entry of a given radionuclide into the body. I have given the dose coefficients and maximum annual intakes of some radionuclides for the population when entering the body through air and food in the table.
Alpha-active isotopes have the greatest radiotoxicity. This is due to the high energy of alpha particles and the high quality factor of alpha particles, equal to 20. At the other end of the scale are tritium and carbon-14, whose decay releases little energy (especially for tritium) and therefore produces a low dose. In addition to the energy of decay, a significant role is played by where this decay occurs. Thus, cesium-137, which is distributed almost evenly throughout the body, has much less radiotoxicity than strontium-90, which concentrates around the bone marrow, or iodine-131, which is almost all concentrated in the thyroid gland.
It is interesting that the radiotoxicity of uranium (especially depleted) can practically be neglected against the background of its chemical toxicity, in which it is comparable to mercury. However, the toxic effects caused by uranium are similar to those caused by radiation: this heavy metal is a mutagen and carcinogen.
A dangerous artifact or a safe toy for a radiophile?
You can often find discussions online about the question: how dangerous is the possession of a particular radioactive item?
Let's figure it out. Various objects with increased radioactivity periodically fall into the hands of “radiophiles”, “radiophobes” and other citizens. Here is a partial list of what I encountered directly:
- Products containing a permanent light composition, usually based on radium-226 - watches, Adrianov compasses, toggle switches, aviation and tank instruments and indicators, marine navigation instruments (in particular, a sextant);
- Uranium glass for various purposes and ceramics coated with uranium-containing glaze;
- Uranium and thorium minerals and products of processing of uranium and thorium ores;
- Thorium-containing lamps, among which the DNP and INP series laser pump lamps, as well as ultra-high-pressure xenon lamps, are especially notable;
- Optics with thorium glass (Japanese Takumar lenses, etc.);
- Heat grids for gas lamps (contain thorium - approximately 1 kBq per grid)
- Smoke detectors containing americium.
The only category of these radioactive artifacts that could noticeably increase the radiation background at a significant distance (more than a couple of tens of centimeters) are products containing SPD. Among them there are very “radiant” specimens. But even among them, I have not met those that it would not be enough to remove from myself at a distance of a meter ( I
have not met, but
there are such people
). To them I can only add a whole case of radioactive Takumars, with which a photo dealer came to a meeting with one of my friends. A meter from this case, the dosimeter alarm reliably went off, showing that the level had exceeded 50 microR/h! All other radioactive objects I found were detected almost closely by the dosimeter and are completely safe in terms of external exposure, unless they are worn in pockets or on the body as jewelry.
But with regard to internal irradiation, only uranium glass, lamps and lenses can be considered completely safe. The radionuclides they contain are reliably isolated in a durable and chemically inert mass of glass or thoriated tungsten (in lamps). And the greatest threat is posed by radioactive light composition. Almost all products containing it are open sources
and very dangerous. In some of them, the SPD is not protected by anything and is applied directly to parts accessible to touch - usually such devices are literally smeared with radium-226. Those who try to open, repair, or remove SPD from them are in greatest danger. A single speck of dust of this light composition that gets into the lungs is highly likely to cause cancer. In addition, the decay of radium in light mass is a powerful source of radon.
Radioactive minerals are also dangerous. Especially those that have an earthy shape, soft and easily destroyed crystals with very perfect cleavage, water-soluble - these are all those uranium micas, otenite and other beauties beloved by collectors. Zircon, monazite, unweathered uraninite are less scary.
Conclusion
Large doses of radiation are guaranteed to make you sick.
You will get sick and the severity of this disease will be determined by how many x-rays you catch. But from smaller doses, even tens of times larger than the maximum permissible limits taken, you will most likely
Nothing will happen.
Nothing at all. You most likely
won't become a superhero, you won't feel worse, you won't get sick more often, you won't age faster, and you won't die prematurely.
The only consequence will be an increased risk
of getting cancer or passing on a bad mutation to your children. And most likely - very small (but proportional to the dose!).
Nevertheless, this risk exists, we should not forget about it, and therefore, if you can avoid getting under the beam, you should not do it.
It is impossible to cover all aspects of radiation safety in one article. I deliberately did not touch upon the topic of radon danger, as well as the topic of criticality and SCR - since I plan to write separate articles about this.
All articles in the series
Radiation: Everyday life of a radiochemical laboratory Radiation: units of measurement Radiation: sources
What can Japanese authorities do to reduce negative health impacts?
Professor Wakeford believes that with quick and correct action by the authorities, the consequences of radiation exposure for the population may be minimal.
The main task, according to Wakeford, should be the evacuation of the population from nearby areas and the prevention of consumption of food products exposed to radiation.
To reduce the risk of radioactive iodine accumulation in the thyroid gland, the population may be given iodine tablets.
In addition, the Japanese diet is rich in iodine, so this may also help combat the effects of radiation.
Are children at greater risk?
Theoretically, yes, since in a young body the process of cell growth and reproduction actively continues. Accordingly, the possibility of deviations from the norm increases in the event of disruption of the normal functioning of cells.
Image copyright BBC World Service Image caption Radiation is especially dangerous for children and their growing bodies
After the Chernobyl disaster in 1986, the World Health Organization recorded a sharp increase in cases of thyroid cancer in children who lived near the nuclear power plant.
The reason for this was the release of radioactive iodine, which accumulates in the thyroid gland.
How dangerous is the situation at the Fukushima nuclear power plant?
At the nuclear power plant itself, ionizing radiation of 400 millisieverts per hour was recorded.
According to radiation specialist Richard Wakeford, professor at the University of Manchester, exposure to radiation of such power is unlikely to lead to the development of radiation sickness. To do this, he said, the irradiation power should be twice as high.
However, even such irradiation can cause a slowdown in the formation of leukocytes in the bone marrow and increases the risk of developing cancer by 2-4%. The average risk of cancer in Japan is 20-25%.
At the same time, Professor Wakeford notes that only those who participated in emergency work at the nuclear reactor were exposed to such radiation. In addition, to reduce the level of exposure, these workers could be involved in work at nuclear power plants only for a short period of time.
The level of exposure of the population, including those living near the nuclear power plant, was much less.
Is it possible to compare the accident at the Fukushima nuclear power plant with the Chernobyl disaster?
As Professor Jerry Thomas, who studied the consequences of the Chernobyl accident, said, it is unlikely that what happened in Japan can be compared with Chernobyl.
“There was an explosion at the Chernobyl nuclear power plant, which completely destroyed the reactor and released a huge amount of radioactive substances into the environment,” says Jerry Thomas.
Professor Thomas emphasizes that the consequences of the Chernobyl accident were mainly observed in those who lived near the nuclear power plant and, mainly, in children.