The production of plastics and most types of rubber is completely unthinkable without the use of special additives - plasticizers. They make the material stronger and more durable, thanks to them they acquire properties such as transparency and flexibility.
Among plasticizers, phthalates occupy the first place. But these chemicals are used not only in industry. In the modern world you can hardly meet a person who is not exposed to them. Let's look at what phthalates are, what harm they cause to health, and how to avoid their negative effects.
Phthalates: what are they?
Phthalates
More than ninety percent of plasticizers are used in the production of polyvinyl chloride, and about ten percent are used in the production of rubber, paint, cosmetics and perfumes.
There are two groups of substances:
- low molecular density;
- high molecular density.
The first group is considered the most toxic and unfavorable for human health.
Industries that produce phthalates have an extremely negative impact on the environment and harm the environment. More than a million tons of plasticizer are produced every year. There are many types of these chemicals, the most popular being the three types of phthalates:
- A very popular variant of the chemical. Often used in the cosmetics industry as a solvent and fragrance fixer, as an alcohol substitute.
- It is often the basis of cosmetic products. Most often used as a component of nail polish.
- Cheap plasticizer with high technological qualities. It is used as an additive in PVC, building materials and perfumes.
Toxic phthalates are most often labeled as fragrances or fragrances on the label.
It is for this reason that people who monitor the quality of purchased products do not find them in the list of components. Manufacturers try to avoid mentioning the chemical.
Table of the most common phthalates
Market trend in decreasing use of low orthophthalates including DEHP
| Name | Abbreviation | Structural formula | Molecular weight (g/mol) | CAS No. |
| Dimethyl phthalate | DMP | C6H4(COOCH3)2 | 194.18 | 131-11-3 |
| Diethyl phthalate | DEP | C6H4(COOC2H5)2 | 222.24 | 84-66-2 |
| Diallyl phthalate | DAP | C6H4(COOCH2CH=CH2)2 | 246.26 | 131-17-9 |
| Di-n-propyl phthalate | DPP | C6H4[COO(CH2)2CH3]2 | 250.29 | 131-16-8 |
| Di-n-butyl phthalate | DBP | C6H4[COO(CH2)3CH3]2 | 278.34 | 84-74-2 |
| Diisobutyl phthalate | DIBP | C6H4[COOCH2CH(CH3)2]2 | 278.34 | 84-69-5 |
| Butyl cyclohexyl phthalate | BCP | CH3(CH2)3OOCC6H4COOC6H11 | 304.38 | 84-64-0 |
| Di-n-pentyl phthalate | DNPP | C6H4[COO(CH2)4CH3]2 | 306.40 | 131-18-0 |
| Dicyclohexyl phthalate | DCP | C6H4[COOC6H11]2 | 330.42 | 84-61-7 |
| Butyl benzyl phthalate | BBP | CH3(CH2)3OOCC6H4COOCH2C6H5 | 312.36 | 85-68-7 |
| Di-n-hexyl phthalate | DNHP | C6H4[COO(CH2)5CH3]2 | 334.45 | 84-75-3 |
| Diisohexyl phthalate | DIHxP | C6H4[COO(CH2)3CH(CH3)2]2 | 334.45 | 146-50-9 |
| Diisoheptyl phthalate | DIHpP | C6H4[COO(CH2)4CH(CH3)2]2 | 362.50 | 41451-28-9 |
| Butyl decyl phthalate | BDP | CH3(CH2)3OOCC6H4COO(CH2)9CH3 | 362.50 | 89-19-0 |
| Di(2-ethylhexyl) phthalate | DEHP, DOP | C6H4[COOCH2CH(C2H5)(CH2)3CH3]2 | 390.56 | 117-81-7 |
| Di(n-octyl) phthalate | DNOP | C6H4[COO(CH2)7CH3]2 | 390.56 | 117-84-0 |
| Diisooctyl phthalate | DIOP | C6H4[COO(CH2)5CH(CH3)2]2 | 390.56 | 27554-26-3 |
| n-Octyl n-decyl phthalate | ODP | CH3(CH2)7OOCC6H4COO(CH2)9CH3 | 418.61 | 119-07-3 |
| Diisononyl phthalate | DINP | C6H4[COO(CH2)6CH(CH3)2]2 | 418.61 | 28553-12-0 |
| Di(2-propylheptyl) phthalate | DPHP | C6H4[COOCH2CH(CH2CH2CH3)(CH2)4CH3]2 | 446.66 | 53306-54-0 |
| Diisodecyl phthalate | DIDP | C6H4[COO(CH2)7CH(CH3)2]2 | 446.66 | 26761-40-0 |
| Diundecyl phthalate | DUP | C6H4[COO(CH2)10CH3]2 | 474.72 | 3648-20-2 |
| Diisoundecyl phthalate | DIUP | C6H4[COO(CH2)8CH(CH3)2]2 | 474.72 | 85507-79-5 |
| Ditridecyl phthalate | DTDP | C6H4[COO(CH2)12CH3]2 | 530.82 | 119-06-2 |
| Diisotridecyl phthalate | DITP | C6H4[COO(CH2)10CH(CH3)2]2 | 530.82 | 68515-47-9 |
The meaning of phthalates
The substances are necessary for the production of cosmetics, toys, plastic and plastic products. They help to impart color and odor resistance, and make the material more resistant to external influences. Plasticizers are difficult to replace, so it is important to reduce their harmful effects on humans.
Read
What are the health benefits of peach?
Scientists have found phthalates to be responsible for increasing the development of such diseases: asthma, attention deficit disorder, breast cancer, diabetes. It is phthalates that interfere with concentration, reduce intellectual activity, and cause infertility or the birth of children with genetic disorders. The main danger of the substance is that it is not bound to the material and can penetrate the walls of the object and enter the air.
Some phthalates are so dangerous that Greenpeace and WHO are trying to ban their use. Not just replace some groups with others, but completely stop using the product. Plasticizers are around us all the time. They are part of the items we use: wallpaper, linoleum, perfume, dishwashing detergent or favorite scent. Even if you find a phthalate-free product, it can simply be “contaminated” from another item. Phthalates are volatile and easily penetrate into a “phthalate-free” product.
Table of the most common phthalates
| Name | Acronym | Structural formula | CAS No. |
| Dimethyl phthalate | DMP | C6H4(COOCH3)2 | 131-11-3 |
| Diethyl phthalate | DEP | C6H4(COOC2H5)2 | 84-66-2 |
| Diallyl phthalate | DAP | C6H4(COOCH2CH=CH2)2 | 131-17-9 |
| Di-n-propyl phthalate | DPP | C6H4[COO(CH2)2CH3]2 | 131-16-8 |
| Di-n-butyl phthalate | DBP | C6H4[COO(CH2)3CH3]2 | 84-74-2 |
| Diisobutyl phthalate | DIBP | C6H4[COOCH2CH(CH3)2]2 | 84-69-5 |
| Butyl cyclohexyl phthalate | BCP | CH3(CH2)3OOCC6H4COOC6H11 | 84-64-0 |
| Di-n-pentyl phthalate | DNPP | C6H4[COO(CH2)4CH3]2 | 131-18-0 |
| Dicyclohexyl phthalate | DCP | C6H4[COOC6H11]2 | 84-61-7 |
| Butyl benzyl phthalate | BBP | CH3(CH2)3OOCC6H4COOCH2C6H5 | 85-68-7 |
| Di-n-hexyl phthalate | DNHP | C6H4[COO(CH2)5CH3]2 | 84-75-3 |
| Diisohexyl phthalate | DIHxP | C6H4[COO(CH2)3CH(CH3)2]2 | 146-50-9 |
| Diisoheptyl phthalate | DIHpP | C6H4[COO(CH2)4CH(CH3)2]2 | 41451-28-9 |
| Butyl decyl phthalate | BDP | CH3(CH2)3OOCC6H4COO(CH2)9CH3 | 89-19-0 |
| Di(2-ethylhexyl) phthalate | DEHP, DOP | C6H4[COOCH2CH(C2H5)(CH2)3CH3]2 | 117-81-7 |
| Di(n-octyl) phthalate | DNOP | C6H4[COO(CH2)7CH3]2 | 117-84-0 |
| Diisooctyl phthalate | DIOP | C6H4[COO(CH2)5CH(CH3)2]2 | 27554-26-3 |
| n-Octyl n-decyl phthalate | ODP | CH3(CH2)7OOCC6H4COO(CH2)9CH3 | 119-07-3 |
| Diisononyl phthalate | DINP | C6H4[COO(CH2)6CH(CH3)2]2 | 28553-12-0 |
| Diisodecyl phthalate | DIDP | C6H4[COO(CH2)7CH(CH3)2]2 | 26761-40-0 |
| Diundecyl phthalate | DUP | C6H4[COO(CH2)10CH3]2 | 3648-20-2 |
| Diisoundecyl phthalate | DIUP | C6H4[COO(CH2)8CH(CH3)2]2 | 85507-79-5 |
| Ditridecyl phthalate | DTDP | C6H4[COO(CH2)12CH3]2 | 119-06-2 |
| Diisotridecyl phthalate | DIUP | C6H4[COO(CH2)10CH(CH3)2]2 | 68515-47-9 |
The harm of phthalates
Asthma
Observations and numerous studies allow us to classify phthalic acid derivatives as mildly toxic substances. Deposition of substances in the human body threatens serious negative reactions. Chronic poisoning is often observed, which leads to the development of malignant neoplasms, hormonal imbalance, asthma, and endocrine diseases.
Young children, people with weakened immune systems and pregnant women are most sensitive to the substance. Chemicals enter the human body through the respiratory tract or skin through prolonged contact with the substance. Upon penetration into the body, a malfunction of the liver, kidneys and respiratory organs occurs. The smell of phthalates can provoke asthma attacks and cause the development of an asthmatic component.
Properties
Phthalate esters are the dialkyl or alkyl aryl esters of phthalic acid (also called 1,2-benzenedicarboxylic acid, not be confused with the terephthalic or isophthalic acids); the name "phthalate" derives from phthalic acid, which itself is derived from the word "naphthalene". When added to plastics, phthalates allow the long polyvinyl molecules to slide against one another. The phthalates have a clear syrupy liquid consistency and show low water solubility, high oil solubility, and low volatility. The polar carboxyl group contributes little to the physical properties of the phthalates, except when R and R' are very small (such as ethyl or methyl groups). Phthalates are colorless, odorless liquids produced by reacting phthalic anhydride with an appropriate alcohol (usually 6- to 13-carbon).
The mechanism by which phthalates and related compounds effect plasticization to polar polymers has been a subject of intense study since the 1960s. The mechanism is one of polar interactions between the polar centers of the phthalate molecule (the C=O functionality) and the positively charged areas of the vinyl chain, typically residing on the carbon atom of the carbon-chlorine bond. For this to be established, the polymer must be heated in the presence of the plasticizer, first above the Tg of the polymer and then into a melt state. This enables an intimate mix of polymer and plasticizer to be formed, and for these interactions to occur. When cooled, these interactions remain and the network of PVC chains cannot reform (as is present in unplasticized PVC, or PVC-U). The alkyl chains of the phthalate then screen the PVC chains from each other as well. They are blended within the plastic article as a result of the manufacturing process.
Because they are not chemically bonded to the host plastics, phthalates are released from the plastic article by relatively gentle means. For example, they can be removed by heating or by extraction with organic solvents.
Alternatives
There are numerous biological alternatives on the market. The problem is that they are typically expensive and not compatible as a primary plasticizer. However, Dioctyl terephthalate (a terephthalate isomeric with DEHP) and 1,2-Cyclohexane dicarboxylic acid diisononyl ester (a hydrogenated version of DINP) are available at cost-competitive pricing and with good plasticization properties.
A plasticizer based on vegetable oil that uses single synthesis reactor and is compatible as a primary plasticizer has been developed. It is a ready substitute for dioctyl phthalate. And several other bio-based plasticizers have been and are being developed as alternatives to phthalates.
Where are phthalates present?
Perfumery
Chemicals can follow us everywhere, they are part of our daily lives and objects that we use every day:
- Perfumery. As an integral part of deodorants and perfumes.
- Children's toys based on PVC. Mostly these are baby dolls and balls. Substances are found even in products for babies: pacifiers, teethers. There is no phthalate odor.
- Cosmetical tools. Nail and hair varnishes that are harmful in themselves contain large quantities of plasticizer. There are also chemicals in shampoos, conditioners, and UV protectants.
- PVC stretch ceiling. Often, very low quality film is used for repairs. In this case, it will release harmful components and poison those living in the house or apartment.
- Plastic PVC windows.
- Finishing materials in the car interior.
- Medical equipment and supplies.
- Packaging materials.
- Much more.
Read
What are the benefits of rice porridge?
This means that it is impossible to completely eliminate contact with phthalates.
Uses
Phthalates are used in a large variety of products, from enteric coatings of pharmaceutical pills and nutritional supplements to viscosity control agents, gelling agents, film formers, stabilizers, dispersants, lubricants, binders, emulsifying agents, and suspending agents. End applications include adhesives and glues, agricultural adjuvants, building materials, personal care products, medical devices, detergents and surfactants, packaging, children's toys, modeling clay, waxes, paints, printing inks and coatings, pharmaceuticals, food products, and textiles. Phthalates are also frequently used in soft plastic fishing lures, caulk, paint pigments, and sex toys made of so-called “jelly rubber.” Phthalates are used in a variety of household applications such as shower curtains, vinyl upholstery, adhesives, floor tiles, food containers and wrappers, and cleaning materials. Personal care items containing phthalates include perfume, eye shadow, moisturizer, nail polish, liquid soap, and hair spray. They are also found in modern electronics and medical applications such as catheters and blood transfusion devices. The most widely-used phthalates are the di-2-ethyl hexyl phthalate (DEHP), the diisodecyl phthalate (DIDP) and the diisononyl phthalate (DINP). DEHP is the dominant plasticizer used in PVC due to its low cost. Benzylbutylphthalate (BBP) is used in the manufacture of foamed PVC, which is mostly used as a flooring material. Phthalates with small R and R' groups are used as solvents in perfumes and pesticides.
As of 2004 manufacturers produced about 363 thousand metric tons (800 million pounds or 400,000 short tons) of phthalates each year. They contribute 10-60% of plastic products by weight.
How to reduce contact with plasticizers
Since contact with phthalates is inevitable, it is necessary to make it less dangerous.
You can still wait a very long time until manufacturers completely abandon the chemical, so you need to try to minimize your contact with phthalates yourself:
- Avoid products in plastic; if this is not possible, do not purchase a product packaged in plastic labeled “three”.
- The product has a high content, which contains flavoring and there is a mention of this on the packaging.
- It is best to buy products in glass.
- Avoid waterproof cosmetics.
- For children and pregnant women, products labeled “phthalate-free” should be purchased.
- Try to purchase toys with the CE mark.
- If a plastic product has a strong odor, discard it.
How to treat phthalic acid poisoning
What to do if you have already been poisoned by phthalates.
Under no circumstances should you leave your child with a toy that emits an unpleasant odor. Children are able to become attached to their favorite things, play, imagine that toys are alive, stay in contact throughout the day: walk, eat, play, and then go to bed together. That is why it is worth making sure that the object your baby adores is, first of all, safe and reliable, otherwise you risk his health, namely poisoning.
If it so happens that you or your child are poisoned by this chemical compound, about which our article is written, then under no circumstances self-medicate! The most logical thing to do is to call an ambulance as soon as possible. Only a therapist who is a professional in his field can help you in this case.
As you understand, such a substance has no advantages, just downsides. In the near future, it is possible that not only some types of phthalic acid will be banned, but also it completely. After all, if Europe has already begun to introduce similar bans, it follows that such problems should also be addressed. Remember that your health depends only on you. Do not believe scammers who are trying to sell you products that can harm you or your child in any way. Protect yourself from counterfeits and low-quality products.
Thank you for your attention! Be healthy))
Research results
Numerous studies on the dangers of phthalates have been ongoing for a long time. At the beginning of the 2000s, American scientists presented the results of their observations. During the work, scientists were able to identify a serious threat from plasticizers to the human body. Despite the conclusion of scientists about the weak toxicity of substances, it can be argued that they are such only if production rules are fully observed.
Study
The main problem and danger is the ability of the substance to accumulate in the human body and poison it. Since substances are not associated with the molecules of objects, they float in the air. One of the most common ways poisons enter the body is inhalation. In addition, it is possible to obtain an unsafe component in the following cases:
- through drinking water;
- through food;
- as a result of prolonged tactile contact.
In the body, the substance is gradually transformed into a metabolite. The product destroys tissue at the cellular level.
The following organs are most affected:
- liver;
- kidneys;
- lungs.
Phthalates in the body can cause hormonal imbalances in men and women. Children experience disorders of puberty. Boys mature much more slowly, with development following the female pattern. In girls, sexual development accelerates.
Read
About vitamins in tangerines
Exposure
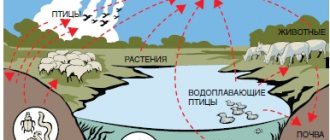
Due to their use as plasticizers, diethyl phthalates are ubiquitous in the environment, especially near places of production and use. Biodegradation through microbially-mediated processes can result in products that can potentially harm microorganisms. There is also general evidence of widespread human exposure. Non-occupational exposure results from the diet, for example phthalate-coated medicines and nutritional supplements, and through consumer products. High occupational exposure was observed in workers directly manufacturing plasticizers. Studies suggest a high correlation between air and urine sample concentrations of short side-chain phthalates such as DEP, making inhalation an important route of exposure.
Healing properties
Conventionally, they can be divided into two significant categories. The first is the effect on physiological health, the second is the psychological aspect. Rubbing into the skin, various therapy against acute respiratory infections and acute respiratory viral infections - this is just the first group.
The second one is worth talking about separately. After all, it is known that oil does not affect everyone equally. Therefore, agarwood is used in perfumery for certain types of products aimed at special clients. Those who love low sound, tart tones, a mixture of woody notes and animalistic scents. So, if you like musk and skin, then, most likely, inhaling the resin will have a beneficial effect on your psychological state.
Do not forget that many sexologists recommend a remedy for increasing libido and potency. And some experts, in principle, consider it a natural aphrodisiac that is very effective.
Toxicity
Little is known about the chronic toxicity of diethyl phthalate, but existing information suggests only a low toxic potential. Studies suggest that some phthalates affect male reproductive development via inhibition of androgen biosynthesis. In rats, for instance, repeated administration of DEP results in loss of germ cell populations in the testis. However, diethyl phthalate doesn't alter sexual differentiation in male rats. Dose response experiments in fiddler crabs have shown that seven-day exposure to diethyl phthalate at 50 mg/L significantly inhibited the activity of chitobiase in the epidermis and hepatopancreas. Chitobiase plays an important role in degradation of the old chitin exoskeleton during the pre-moult phase.
Teratogenicity
When pregnant rats were treated with diethyl phthalate, it became evident that certain doses caused skeletal malformations, whereas the untreated control group showed no resorptions. The amount of skeletal malformations was highest at highest dose. In a following study it was found that both phthalate diesters and their metabolic products were present in each of these compartments, suggesting that the toxicity in embryos and fetuses could be the result of a direct effect.
Identification in plastics
Some type 3 plastics may leak phthalates.
Phthalates are used in some but not all PVC formulations, and there are no labeling requirements for phthalates specifically. PVC plastics are typically used for various containers and hard packaging, medical tubing and bags, and are labeled “Type 3” for recycling reasons. However, the presence of phthalates rather than other plasticizers is not marked on PVC items. Plasticizers such as phthalates are only used for making flexible PVC, so it stands to reason that they should only be present in soft forms of PVC. If a more accurate test is needed, chemical analysis, for example by gas chromatography, can establish the presence of phthalates.
Structure and reactivity
Diethyl phthalate, or o-Benzenedicarboxylic acid diethyl ester consists of a benzene ring with two carboxylic acid ethyl esters attached to it in the ortho (1,2) pattern. It is a highly conjugated system, as the pi-cloud on the benzene ring, the p-orbitals on the carbonyl atoms and the lone pairs on the oxygens are all conjugated. The substituents are meta-directing, and they are ortho to each other, so all positions in the ring are more or less equally deactivated. Diethyl phthalate is likely to undergo biodegradation in the environment. Abiotic degradation processes such as hydrolysis, oxidation, and photolysis are unlikely to play significant roles in the environmental fate of diethyl phthalate.
Biodegradation
Biodegradation by microorganisms
Biodegradation of DEP in soil occurs by sequential hydrolysis of the two diethyl chains of the phthalate to produce monoethyl phthalate, followed by phthalic acid. This reaction occurs very slowly in an abiotic environment. there exists an alternative pathway of biodegradation which includes transesterification or demethylation by microorganisms, thus the soil is also contaminated with methanol, that would produce another three intermediate compounds, ethyl methyl phthalate, dimethyl phthalate and monomethyl phthalate. This biodegradation has been observed in several soil bacteria. Some bacteria with these abilities have specific enzymes involved in the degradation of phthalic acid esters such as phthalate oxygenase, phthalate dioxygenase, phthalate dehydrogenase and phthalate decarboxylase. The developed intermediates of the transesterification or demethylation, ethyl methyl phthalate and dimethyl phthalate, enhance the toxic effect and are able to disrupt the membrane of microorganisms.
Biodegradation by mammals
Recent studies show that DEP, a phthalic acid ester (PAE), is enzymatically hydrolyzed to its monoesters by pancreatic cholesterol esterase (CEase) in pigs and cows. These mammalian pancreatic CEases have been found to be nonspecific for degradation in relation to the diversity of the alkyl side chains of PAEs. .
Oud perfumery, what is the secret of its high cost
Actually, this is not a secret. As we said at the beginning, there are several logical arguments.
• A long process of growing the tree is required. At least a hundred years old. That is, it is impossible to artificially increase turnover and supply in the near future.
• Complex mining process. After all, not every plant reacts equally to fungal infection; some produce more resin, others less.
• Huge consumption. As has already been clarified, a mini bottle of extract requires tens of kilograms of raw materials. And the best, the core.
Although, the cost also depends on the seller. For example, in the AromaCode project, thanks to its loyal pricing policy, you can purchase perfumes with notes of agarwood at a completely logical and affordable price. In comparison with analogues, of course.
Legal status
Canada
In 1994, a Health Canada assessment found that DEHP and another phthalate product, B79P, were harmful to human health. The Canadian federal government responded by banning their use in cosmetics and restricting their use in other applications.
A more recent assessment in 2020 found that B79P and DEHP may cause environmental damage. As of 2020, regulations to protect the environment against DEHP and B79P have not yet been put into place.
European Union
Update on Non-Classified plasticisers and the European REACH Candidate Classification including pending authorization
The use of some phthalates has been restricted in the European Union for use in children's toys since 1999. DEHP, BBP, and DBP are restricted for all toys; DINP, DIDP, and DNOP are restricted only in toys that can be taken into the mouth. The restriction states that the amount of these phthalates may not be greater than 0.1% mass percent of the plasticized part of the toy. Generally the high molecular weight phthalates DINP, DIDP and DPHP have been registered under REACH and have demonstrated their safety for use in current applications. They are not classified for any health or environmental effects.
The low molecular weight products BBP, DEHP, DIBP, and DBP were added to the Candidate list of Substances for Authorization under REACH in 2008-9, and added to the Authorization list, Annex XIV, in 2012. This means that from February 2020 they are not allowed to be produced in the EU unless authorization has been granted for a specific use, however they may still be imported in consumer products. The creation of an Annex XV dossier, which could ban the import of products containing these chemicals, is being jointly prepared by the ECHA and Danish authorities and is expected to be submitted by April 2020.
The Dutch office of Greenpeace UK sought to encourage the European Union to ban sex toys that contained phthalates.
United States
During August 2008, the United States Congress passed and President George W. Bush signed the Consumer Product Safety Improvement Act (CPSIA), which became public law 110-314. Section 108 of that law specified that as of February 10, 2009, “it shall be harmful for any person to manufacture for sale, offer for sale, distribute in commerce, or import into the United States any children's toy or child care article that contains concentrations of more than 0.1 percent of" DEHP, DBP, or BBP and "it shall be harmful for any person to manufacture for sale, offer for sale, distribute in commerce, or import into the United States any children's toy that can be placed in a child's mouth or child care article that contains concentrations of more than 0.1 percent of» DINP, DIDP, DnOP. Furthermore, the law requires the establishment of a permanent review board to determine the safety of other phthalates. Prior to this legislation, the Consumer Product Safety Commission had determined that voluntary withdrawals of DEHP and DINP from teethers, pacifiers, and rattles had eliminated the risk to children, and advised against enacting a phthalate ban.
In another development in 1986, California voters approved an initiative to address their growing concerns about exposure to toxic chemicals. That initiative became the Safe Drinking Water and Toxic Enforcement Act of 1986, better known by its original name of Proposition 65. In December 2013 DINP was listed as a chemical “known to the State of California to cause cancer” This means that, starting December 2014, companies with 10 or more employees manufacturing, distributing or selling the product(s) containing diisononyl phthalate (DINP) are required to provide a clear and reasonable warning for that product. The California Office of Environmental Health Hazard Assessment, charged with maintaining the Proposition 65 list and enforcing its provisions, has implemented a “No Significant Risk Level” of 146 ug/day for DINP, as of April 1, 2020.
Metabolism
Diethyl phthalate is hydrolyzed to monoester, monoethyl phthalate and ethanol after oral administration in the lumen of the gastrointestinal tract or in the intestinal mucosal cells. Hydrolysis of DEP also takes place at the kidney and liver after systemic absorption. After tissue distribution throughout the body, DEP accumulates in the liver and kidney. The metabolites are excreted in the urine. DEP is metabolized by carboxyl esterase, which is synthesized in the human liver. In vitro studies show that DEP reduces the glucuronyl transferase activity. It was also observed that the activity of peroxisomal enzyme carnitine acetyl transferase is increased in cultures of rat liver cells. Furthermore, DEP induces the enzyme activity of catalase, which leads to hepatic peroxisome proliferation and possibly causes hyperplasia.
Properties
Phthalate esters are the dialkyl or alkyl aryl esters of phthalic acid (also called 1,2-benzenedicarboxylic acid, not be confused with the isomeric terephthalic or isophthalic acids); the name phthalate derives from phthalic acid, which itself is derived from the word “naphthalene”. When added to plastics, phthalates allow the long polyvinyl molecules to slide against one another. The phthalates have a clear syrupy liquid consistency and show low water solubility, high oil solubility, and low volatility. The polar carboxyl group contributes little to the physical properties of the phthalates, except when R and R' are very small (such as ethyl or methyl groups). They are colorless, odorless liquids produced by reacting phthalic anhydride with an appropriate alcohol (usually 6 to 13 carbon).
Main Applications
The plasticizer DBP dibutyl phthalate finds its application in the plastics industry. It is used in the manufacturing process:
- • paint and varnish products,
- • polyvinyl chloride,
- • cellulose ethers,
- • polyacrylates,
- • polyurethane sealants,
- • nitrile rubber,
- • artificial film materials (various polyethylene),
- • linoleum,
- • polyvinyl acetate compounds,
- • cable plastic,
- • artificial resin,
- • rubber,
- • “cold” porcelain.
The addition of dibutyl phthal is due to the fact that it increases the wear resistance of the material, prevents cracks, improves the appearance of the product, adds shine, and increases strength.
Identification in plastics
Some type 3 plastics may leach phthalates.[ citation needed
]
Phthalates are used in some but not all PVC formulations, and there are no specific labeling requirements for phthalates. PVC plastics are typically used for various containers and hard packaging, medical tubing, and bags, and are labeled “Type 3” for recycling reasons. However, the presence of phthalates rather than other plasticizers is not marked on PVC items. Only unplasticized PVC (uPVC), which is mainly used as a hard construction material, has no plasticizers. If a more accurate test is needed, chemical analysis, for example by gas chromatography or liquid chromatography, can establish the presence of phthalates.
Polyethylene terephthalate (PET, PETE, Terylene, Dacron) is the main substance used to package bottled water and many sodas. Products containing PETE are labeled “Type 1” (with a “1” in the recycle triangle) for recycling purposes. Although the word “phthalate” appears in the name, PETE does not use phthalates as plasticizers. The terephthalate polymer PETE and the phthalate ester plasticizers are chemically different substances. Despite this, however, a number of studies have found phthalates such as DEHP in bottled water and soda. One hypothesis is that these may have been introduced during plastics recycling.
Perfume with agarwood scents - TOP 7 best
Now let’s take a brief look at the most famous and popular representatives of perfumery, who have already managed to add agar resin to their set of best ingredients.
Oud Ispahan, Dior
Can you imagine a very languid and powerful Damascus rose and an even more overwhelming base of viscous oud? But Dior did it. And in his brainchild, viscous motifs from eastern countries sound so strongly that in hot weather they can be a little suffocating. Therefore, it is recommended for coolness in the off-season.
Oud Wood, T.F.
As already noted, agarwood, in principle, contains its own bitterness and pungency, which, however, not everyone can detect. But here everyone can definitely do it, because there is a huge amount of pepper in the perfume. To be honest, the best oud scents are often the ones that are uncompromising. And this is exactly such an option. Powerful, bright, bitter. Sink or swim.
Oud Immortel, Byredo
But this is exactly that fresh and atypical and somewhat adventurous option. Herbal tones, a little sharp citrus, a little fruity freshness. Original and very mobile, active, easy. Which, in principle, is strangely reflected against the background of such a base.
Red Aoud, Montale
Spicy, haunting, very long lasting, with an endless trail. In fact, the description fits most of the products from this brand. Everyone knows how long-lasting elixirs from the manufacturer are. But the oud aromas of Montal are the quintessence of a deep and languid taste, from which it is difficult to abstract. It does not single out a person, but gives him a new image.
Oud, M.F.K.
We have repeatedly emphasized that not everyone will be able to cope with melodies based on agar resin. It’s heavy, it smells like sex, it’s oppressive and doesn’t allow you to make out anything else in the background. But in this case, the manufacturer simply created something great. Taking all the best from oud, the resulting product was immersed in sweet pepper. Sometimes it becomes really difficult to breathe. But it is almost impossible to confuse such a smell with something else. He is unique.
Amber Aoud, RD
The purest unisex, which powerfully attacks with moss, birch bark, morning dew and fresh grass. And on the base of sandalwood, musk is revealed so precisely that even connoisseurs will not be able to determine the presence of oud. But it exists and is woven into this cacophony at the very bottom, setting the tone for the entire movement. A natural and fresh solution from a perfumer.
Rose Oud, Kilian
And finally, a very interesting approach. Here you can find both rose tincture and rose water. And such an undisguised line of colors without any transitions is placed directly on the wood base. It turns out very modest, limited. But at the same time, both tastes are not shaded, they play brightly, loudly and, interestingly, for a long time.
Hazard Class
Dibutyl phthalate is a highly hazardous substance that can form life-threatening concentrations even with minor leaks. According to GN (Hygienic Standards) 2.2.5.1313-03 dated April 27, 2003 “Maximum permissible concentrations (MPC) of harmful substances in the air of the working area,” the maximum permissible indicator for Dibutyl phthalate (DBP/DBP/Dibutylbenzene-1,2-dicarbonate) is 1 .5/0.5 mg/m³*, which is typical for substances of the second hazard class. (*1.5 mg/m³ maximum single dose)
- In Europe, in July 2005, a resolution was passed banning the use of Dibutyl phthalate in children's products.
- In the US, the use of DBP is already almost completely banned in children's toys.
- In Taiwan, the use of dibutyl phthalate was banned almost immediately after Europe
- In Canada, DBP was included in the list of priority candidates for the assessment and certification of chemicals under the European Union REACH initiative
- In the US, major cosmetics companies (L'Oreal and Revlon) have committed to eliminating DBP from their cosmetic products.